It07:Halomon
Halomon
| It07:Halomon | |
|---|---|
| |
| Identifiers | |
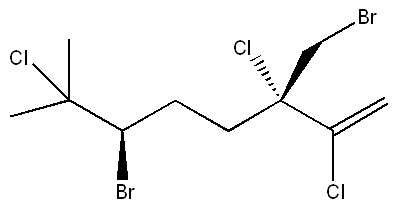
| Systematic name | (3S,6R)-6-Bromo-3-(bromomethyl)-2,3,7-trichloro-7-methyl-1-octene |
| Molecular formula | C10H15Br2Cl3 |
| SMILES | ClC(C)([C@H](Br)CC[C@](CBr)(Cl)C(Cl)=C)C |
| Molar mass | 401.4 gmol-1 |
| CAS number | 142439-86-9 |
| Properties | |
| Melting point | 329-330 K |
| Density | 1.824 g/cm 3 |
| Optical Rotation | -40.2 [alpha] degrees at 589nm in 0.75g/100ml dichloromethane |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox disclaimer and references | |
Halomon was first isolated from red algae (Portieria hornemannii), as a major part of organic extractions containing several chemical compounds, which overall may seem to be a defence against sea life that see it as a source of food.
Halomon is a polyhalogenated acyclic monoterpene. Halocarbons are generally good alkylating agents and can be toixic to cells and organisms, however halomon shows selective cytoxicity, and so has potential for safe use.
Jmol 3D structural views of molecule:
The above buttons can be used to modify the viewing mode of the jmol, one of which includes a 3D view which can be seen with Blue/Red 3D glasses.
Potential in Anti-Caner Research
Halomon was screened though an anti-HIV assay, where it was found to be inactive. However the results did show cytotoxic effects against uninfected human lymphoblastoid control cells, which were used as part of the AIDS-antiviral screen. Halomon was then tested in an in vitro human tumor screen, where it showed a range of selective cytotoxicity against a broad range of tumors. The research showed that several brain, renal and colon tumors were more sensitive than certain less sensitive melanoma and leukaemia lines. In some cases as much as 1000 or more times more at the GI50 level and 100 times or more at the LC50 (lethal concentration that kills 50%) level. Halomon’s selective cytotoxicity to certain kinds of tumor cells mean that it has great potential (as part of a cocktail or its derivatives) in cancer treatment.[1]
As a result, the NCI (National Cancer Institute) decision network committee selected the compound for preclinical drug development . However despite this, and encouraging in vivo results, this further development and research is constrained by the small amounts of Halomon available naturally, due to lack of synthetic methods. Research by Richard W. Fuller et alii shows research on ten halogenated monoterpenes related to halomon [2], and research on mice of the pharmacokinetics and tissue distribution of halomon have been carried out by M.J. Egorin et alii.[3]
A more recent paper (2006) by Eric H. Andrianasolo et alii shows polyhalogentaed monoterpenes (including halomon) inhibit the enzyme DNA methyltransferase, giving further information and understanding on the pharmacological pathways used by the compound.[4]
Synthesis Reasearch
Due to the potential of this compound, there is good interest in finding potential synthetic routes for the synthesis of this compound. One early (1998) method (Thierry Schlama et alii) uses a Johnson-Claisen rearrangement.[5]
A diagramatic representation taken from the published journal is shown below:
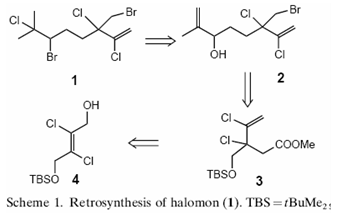
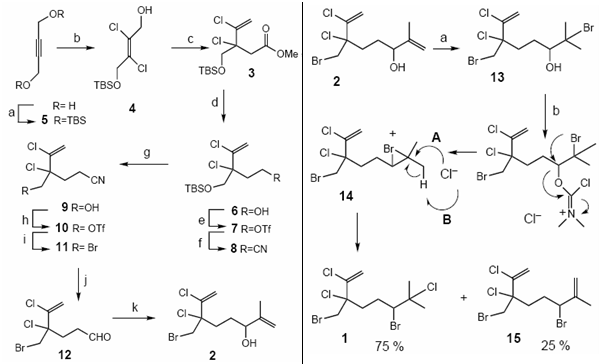
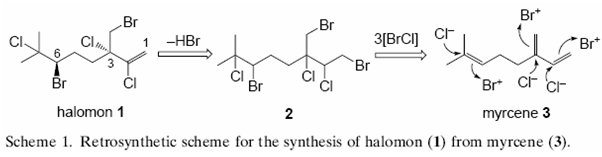
A second, more recent method (2000) by Masahiro Hiroma et alii, uses a three step process:
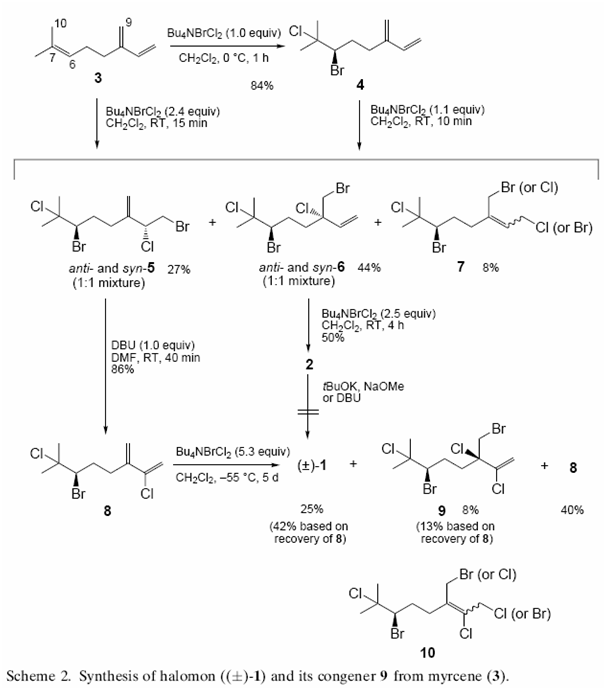
References
- ↑ 1. R. W. Fuller, J. H. Cardellina, Y. Kato, L. S. Brinen, J. Clardy, K. M. Snader and M. R. Boyd, J. Med. Chem., 1992, 35, 3007-3011.
- ↑ 1. R. W. Fuller, J. H. Cardellina, J. Jurek, P. J. Scheuer, B. Alvarado-Lindner, M. McGuire, G. N. Gray, J. R. Steiner, J. Clardy and et al., J. Med. Chem., 1994, 37, 4407-4411.
- ↑ 1. M. J. M. J. Egorin, Cancer chemotherapy and pharmacology, 1996, 39, 51-60.
- ↑ 1. E. H. E. Andrianasolo, Journal of natural products, 2006, 69, 576.
- ↑ 1. R. B. V. G. A. V. J. R. F. C. M. Thierry Schlama, Angewandte Chemie International Edition, 1998, 37, 2085-2087.
- ↑ 1. R. B. V. G. A. V. J. R. F. C. M. Thierry Schlama, Angewandte Chemie International Edition, 1998, 37, 2085-2087.
- ↑ 1. R. B. V. G. A. V. J. R. F. C. M. Thierry Schlama, Angewandte Chemie International Edition, 1998, 37, 2085-2087.
- ↑ 1. T. T. Sotokawa, Angewandte Chemie (International ed.), 2000, 39, 3430.
- ↑ 1. T. T. Sotokawa, Angewandte Chemie (International ed.), 2000, 39, 3430.
