It07:Glutamic acid
Glutamic acid
Glutamic acid is the conjugate acid of glutamate. Due to its two carboxylic acid groups and an amine group it is said to have an amino-acid structure. It is one of the common 20 amino-acids and is required in the body but is not an "essential" amino acid. Due to the nature of one basic and two acidic groups it is easier to state the isoelectric point(pI) of the molecule as 4.20 as oppose to the pKas of the molecule. In the body glutamate can be used as a neurotransmitter and also as a respiritory substrate, both of which are extremely important to the human body.
3D Structure
Single Molecule
| Glutamic acid | |
|---|---|

| |
| General | |
| Systematic name | 2-Amino-pentanedioic acid |
| Other names | glutamic acid, DL-Glutaminsaeure, DL-glutamic acid |
| Molecular formula | C5H9NO4
|
| SMILES | OC(CCC(N)C(O)=O)=O |
| Molar mass | 147.13 |
| CAS number | 56-86-0, 617-65-2, 6893-26-1, 6899-05-4 |
| Properties | |
| Density & phase | 1.593 g/cm³ [1] |
| Solubility in water | 0.0937 mol/kg [2](25°C) |
| Melting point | 199°C |
| Isoelectric point (pKI) | 4.20 [3] |
| Chiral rotation [α]D | 30° 589nm 20°[4] |
| Structure | |
| Crystal structure | monoclinic: beta=103.7 grad, a=5.74 Å, b=13.04 Å, c=8.43 Å. [5] |
| Dipole moment | Varies on conformation D |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox disclaimer and references | |
Ammonium Glutamate unit cell
Showing packing in a crystal
Ammonium Glutamate |
Neurotransmitter
Glutamate acts in the brain to open ion-channels to allow the flow of positive ions through a synaptic membrane. This means that it is always and excitatory neurotransmitter. An interesting point about the receptor for glutamate (NDMA glutamate receptor) is that it is the only known receptor to be regulated by potentials and by binding. This receptor is controlled by the use of five binding sites one each for:
the NMDA receptor is also thought to be involved in some types of memory and learning. Ethanol is thought to inhibit NMDA activities and hence the effects of "alcohol" on the brain. These receptor are thought to be found mainly in the hippocampus, amygdala and basal ganglia. In large amounts glutamate can actually damage brain neurones. Monosodium glutamate (large amounts in soy sauce) has been shown to destroy brain cells in young animals. It is not usually considered to cross the barrier between the blood and the brain but is a possibility in humans. Caffeines increase in awareness could be due to the blockage of the receptors which usuaully prevent release of the glutamate.
[7]Glutamate is however much more than just a standard neuro transmitter. It can not only activate neuroactivity but can also dampen or reinforce current activity. Also release of glutamate from Astrocytes is dependant on the metabolism of the cells themselves which is very unique. GABA (an inhibitor), for which Glutamate is a precursor, comes the closest to this method of release. Here is a summary of the important facts that make glutamic acid different:
- Astrocytes relase Glutamate using vesicular exocytosis using proteins VGLUT1 and VGLUT2
- A lot of regulatory measurements in Astrocytic cells regulate the re-release of glutamate. Glutamate can be used for GABA production also.
- De novo (from simple molecules) synthesis of glutamate comes from 20% of glucose metabolism in the brain. It is only recycled once or twice before destoyed and a new glutamate made.
- Glutamate in tissues during glutamate usage actually increases because de novo synthesis exceeds being broken down.
Spectroscopy
IR spectrum

Mass spectrum

UV/Visible spectrum

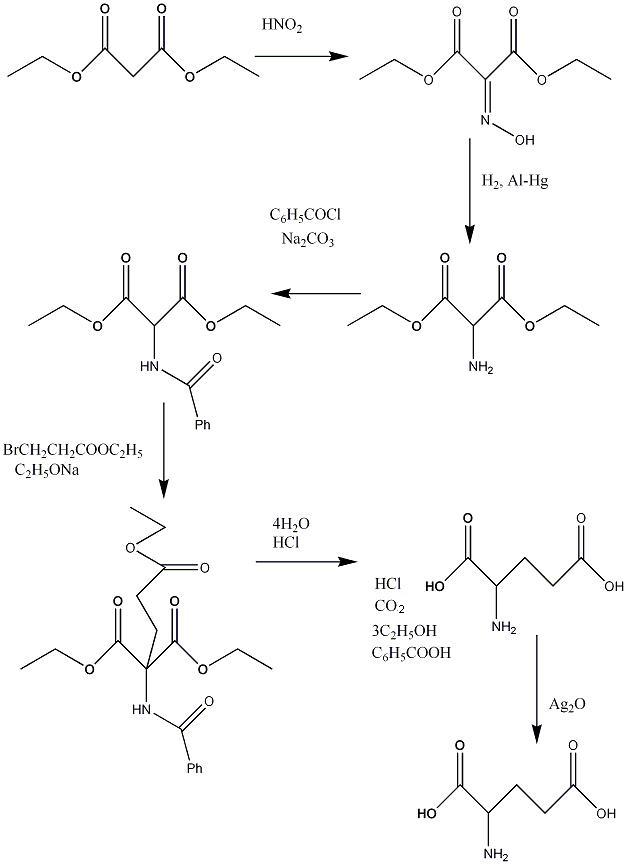
Synthesis
adapted from Journal of biological chemistry [11]
Reaction involved in the synthesis can be seen below. The reactions involve simple Sn2 displacements aswell as simple ketone chemistry up until the penultimate step. The last two steps simply involve hydrolysis and then removal of byproducts by various methods. The Silver oxide is simply for the removal of chloride ions from the solution.
Glutamate dehydrogenase (use in respiration)
This enzyme is involved in the conversion of Glutamate to α-Ketogluterate and visa versa. It is found in the mitochondira of eukaryotic cells and when there is a low level of glucose, glutamate is converted to α-Ketogluterate. This can then be put into the krebs cycle and ultimately will end in the production of ATP. For either forward or backward reactions there are additional chemicals needed for the conversion between the two. For conversion to α-Ketogluterate NAD+ is needed and for the reverse reaction NADP+ is required. [12]
Glutamate dehydrogenase |
References
- ↑ J. D. Dunitz and W. B. Schweizer, Acta Crystallographica Section C, 1995, 51, 1377-1379.DOI:10.1107/S0108270195001648
- ↑ A. Apelblat and E. Manzurola, The Journal of Chemical Thermodynamics, 1997, 29, 1527-1533.DOI:10.1006/jcht.1997.0267
- ↑ H. C. M. A. Sebastian Wohlrab, Angewandte Chemie, 2005, 117, 4156-4161.DOI:10.1002/ange.200462467
- ↑ L. Fowden and A. Smith, Phytochemistry, 1968, 7, 809-819.DOI:doi:10.1016/S0031-9422(00)84836-0
- ↑ J. D. Dunitz and W. B. Schweizer, Acta Crystallographica Section C, 1995, 51, 1377-1379.DOI:doi:10.1107/S0108270195001648
- ↑ World of Ben Best Science http://www.benbest.com/science/anatmind/anatmd10.html#glutamate
- ↑ 1. L. Hertz, Neurochemistry International, 2006, 48, 416-425. DOI:10.1016/j.neuint.2005.12.021
- ↑ NIST Chemistry WebBook, NIST Standard Reference Database Number 69, June 2005 Release, http://webbook.nist.gov/cgi/cbook.cgi?ID=C56860&Units=SI&Mask=80#IR-Spec
- ↑ NIST Chemistry WebBook, NIST Standard Reference Database Number 69, June 2005 Release, http://webbook.nist.gov/cgi/cbook.cgi?Spec=C56860&Index=0&Type=Mass&Large=on
- ↑ NIST Chemistry WebBook, NIST Standard Reference Database Number 69, June 2005 Release, http://webbook.nist.gov/cgi/cbook.cgi?ID=C56860&Units=SI&Mask=400#UV-Vis-Spec
- ↑ Max S. Dunn, B.W. Smart, C.E. Reedman. "A new synthesis of glutamic acid" Journal of Biological Chemistry 92(2): 599-609, September 1931.
- ↑ Plaitakis, A. and I. Zaganas (2001). "Regulation of human glutamate dehydrogenases: Implications for glutamate, ammonia and energy metabolism in brain." Journal of Neuroscience Research 66(5): 899-908.