It07:Gingerone
| Zingerone | ||
|---|---|---|
| ||
| General | ||
| Systematic name | (4-(4'-hydroxy-3'-methoxyphenyl)-2-butanone) | |
| Other names | ginger ketone, gingerone, 4-hydroxy-3-methoxybenzyl acetone, 2-(4-hydroxy-3-methoxyphenyl ethyl) methyl ketone, 4-(4-hydroxy-3-methoxyphenyl) butan-2-one, (4-hydroxy-3-methoxyphenyl) ethyl methyl ketone, 2-(4-hydroxy-3-methoxyphenyl) ethyl methyl ketone, 4-(4-hydroxy-3-methoxyphenyl)-2-butanone, 4-hydroxy-3-methyoxyphenyl ethyl methyl ketone, 3-methoxy-4-hydroxybenzyl acetone, 4-(3-methoxy-4-hydroxyphenyl)-2-butanone, 1-(3-methoxy-4-hydroxyphenyl)-3-butanone, vanillyl acetone, zingherone, zingiberon | |
| IUPAC Name | 4-(4-hydroxy-3- methoxyphenyl)-2-butanone | |
| Molecular formula | 4-(HO)C6H3-3-(OCH3)CH2CH2COCH3 | |
| Empirical Formula | C11H14O3 | |
| SMILES | OC1=C(OC)C=C(CCC(C)=O)C=C1 | |
| Molar mass | 194.23g | |
| Appearance | yellow to yellow brown crystals | |
| Organoleptic [1] | clove; creamy; spicy; sweet; animal | |
| CAS number | 122-48-5 | |
| Properties | ||
| Density & phase | 1.111 ± 0.06g/cm³ | |
| Refractive Index[2] | n20/D 1.541 | |
| Melting point | 40.00 - 41.00 °C. @ 760.00 mm Hg | |
| Boiling point | 141.00 - 0.50 °C. @ 0.00 mm Hg
290.00 °C. @ 760.00 mm Hg | |
| MSDS Data Sheet | [3] | [1] |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox disclaimer and references | ||
Introduction
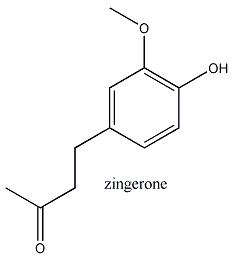
Zingerone(4-(4'-hydroxy-3'-methoxyphenyl)-2-butanone) is a natural molecule best known for providing the hot flavour of the spice ginger and is also present in raspberries and cranberries. The compound is not present in raw ginger, but is converted from the compound gingerol ((S)-5-hydroxy-1-(4-hydroxy-3- methoxyphenyl)-3-decanone) during the cooking process.
The prefix “Zinger” stems from the latin for ginger "zingiber". “-one” is the suffix associated with the ketone functional group.
Zingerone 3D Structure
Zingerone as an antioxidant
Zingerone can act as an antioxidant against peroxynitrile. This is of interest because peroxynitrile in the human body can cross cell membranes to react with the lipids, DNA and proteins of the cell, ultimately causing cell death.
Zingerone as a medicine
Ginger has been regarded as one of traditional methods for curing colds. Nowadays for medicine, it has been developed and used to treated various medical problems. Zingerone reacts with free radicals that can cause tissue damage and inflammation.At Case Western University, research has been done showing that a topically applied extract containing zingerone may help prevent some skin cancers. In capsule form, ginger can also be used to replace anti-inflammatory drugs. In a recent study, ginger was found to be more effective than drugs in the treatment of nausea and motion sickness. Zingerone also has a major role in lipid oxidation since it is an anti-oxidant. It weakly inhibits oxidation of phospholipid liposomes in the presence of iron (III) and ascorbate to prevent heart-attacks.[4]
Synthesis
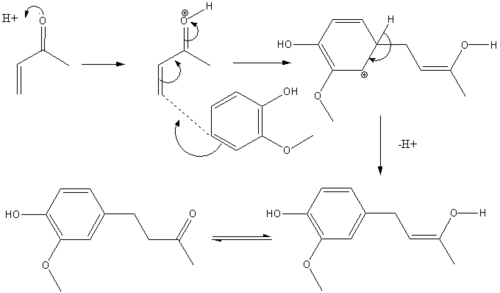
Bunce and Reeves Method
Another synthetic method is described by Bunce and Reeves [2] where a phenol reacts with an α,β-unsaturated ketone. The mechanism is reported to be a Michael type addition where the reactant enone is activated first by protonation. However the yield of this process is rather poor at 38%. This can be attributed to hydrogen bonding between the methoxy oxygen and the phenol. The H bonding ties up the lone pair on the methoxy oxygen leaving it less able to donate into the ring. This in turn renders the ring less nucleophilic and so less able to add to the β position of the enone in the Micaheal addition.
Mechanism
Zingerone stems from vanillin and can be synthesised in the following way[3] -
The first known synthesis of Zingerone was completd by Nomura and reported in 1917.Cite error: Closing </ref> missing for <ref> tag
One particular plant that uses Zingerone in this way is the orchid of the species Bulbophyllum patens that grows in the Malaysian rainforests.

The plant has a single flower that excretes Zingerone to attract fruit flies of the species B. carambolae, B. caudata, B. cucurbitae, B. tau, and B. umbrosa. As the fly lands to feed on the Zingerone, it is temporarily trapped between the hinged see-saw lip of the flower and its column. On escaping the fly removes the flower's pollinarium which is then transferred to another plant during pollination. Zingerone is not only found on the lip but in all parts of the flower.
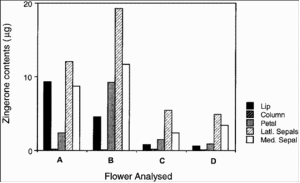
In this way it may help deter predators, wary of Zingerone's fiery taste.
The Zingerone is also useful to the fruit fly. Males are known to store it in their rectal glands to either excrete it as a pheromone mist to attract prospective females or to convert it to Zingerol for the same purpose. On a less carnal note the Zingerone, that is a mild irritant and tastes hot, plays an important defensive role in warding off potential predators.
Spectra
Mass Spectrum[5]
IR Spectrum[6]
13C NMR[7]
References
- ↑ Sigma Aldrich
- ↑ Bunce, Richard A. and Reeves, Henry D. (1989) 'Amberlyst-15 Catalyzed Addition of Phenols to α,β-Unsaturated Ketones', Synthetic Communications, 19:5, 1109 - 1117
- ↑ D. S. D. Kim, Bioorganic & medicinal chemistry letters, 2004, 14, 1287.
- ↑ http://lh3.google.com/_lC8IaiYQP7A/RwNxtOnHOCI/AAAAAAAABzM/M_TsUYA5WyI/s800/bulbophyllum+patens+hbl+913609+borneo.jpg%7Ccentre
- ↑ http://webbook.nist.gov/cgi/cbook.cgi?ID=C122485&Units=SI&Mask=200#Mass-Spec
- ↑ http://webbook.nist.gov/cgi/cbook.cgi?ID=C122485&Units=SI&Mask=80#IR-Spec
- ↑ WSS: Spectral data were obtained from Wiley Subscription Services, Inc. (US)