It07:Ethylene Glycol
| It07:Ethylene Glycol | |
|---|---|
| |
| General | |
| Systematic name | Ethane-1,2-diol |
| Other names | 1,2-dihydroxyethane,Ethylene Glycol, 1,2-ethanediol, EG, glycol, glycol alcohol, monoethylene glycol, ethylene alcohol |
| Molecular formula | C2 H6 O2 |
| SMILES | OCCO |
| Molar mass | 62.068 g/mol |
| Appearance | colourless viscous liquid |
| CAS number | 107-21-1 |
| Properties | |
| Density & phase | 1.1132 g/cm³ |
| Solubility in water | Miscible in all proportions of water |
| Melting point | 260.25 K |
| Boiling point | 470.45 K |
| Viscosity | 16.1 mPa s |
| Hazards | |
| MSDS | [1]MSDS File (html)] |
| Main hazards | Harmful if swallowed, inhaled or in contact with the skin. Skin and respiratory irritant. Severe eye irritant. Typical OEL 10 - 25 ppm. Reproductive hazard. |
| Flash point | 110oC |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox disclaimer and references | |
Ethylene Glycol(MEG) is clear, colourless liquid diol with the consistency of syrup. It is used primarily as a coolant, in polyester manufacturing as well as various other uses. Its production dates back to 1859 and can be a potential health hazard due to its toxicity.
3D Molecular Structure
Ethylene Glycol |
History
Ethylene Glycol was first prepared by Wurtz in 1859 initially by saponifaction with potassium hydroxide of ethylene glycol diacetate and then later in 1860 from hydration of ethylene oxide . In 1917 ethylene glycol was then produced from ethylene chlorohydrin. The first reported use was in World War I as it was used in the explosives industry as a substitute for glycerol. The first glycol commercial plant was set up in 1925 by Carbide and Carbon chemicals, supplying primarily dynamite manufacturers. It wasn’t until 1930 that ethylene glycol , under the name Prestone, was sold as antifreeze. In 1930 Dow started the large- scale production of ethylene glycol using ethylene chlorohydrins and in 1940 Dupont set up an ethylene glycol plant which produced via a formaldehyde methanol process .[1]
Production
Ethylene glycol can be produced by the reaction of ethylene oxide with water at 195oC under pressure. Alternatively it may be produced by the reaction of sodium hydrogen carbonate with ethylene chlorohydrin. In 2005 it was reported that 18 million tonnes of ethylene glycol was produced globally. [2][3]
Commercial Uses
Antifreeze
Ethylene glycol is used in vehicle cooling systems as part of an antifreeze mixture. Initially alcohols were used due to the fact that they were stable and not corrosive however due to their relatively low boiling points they were not ideal for operating at engine temperatures. Ethylene glycol however can operate at high engine temperatures as it has a higher boiling point and in a mixture with water will freeze at -51oC.[4].
Polyester manufacturing
Ethylene glycol is used in polyester manufacturing as a diol. It is reacted with terephthalic acid via an esterification reaction , producing the ethylene terephthalate monomer . This is followed by polycondensation reactions of the monomers to produce PET(polyethylene terephthalate).PET is used in resins and films as well as in fibre manufacturing.

|
| Synthesis of PET |
Other uses
- Photographic developing solutions
- Hydraulic brake fluids
- Ink for stamp pads
- Solvents for paint industry
- Dessicant for natural gas production
- Manafacture of vaccines
- Preventative and treatment of rot for wood.
[6].
Health Hazards
.
Ethylene Glycol Poisoning
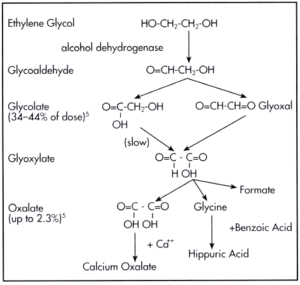
If ingested, ethylene glycol can cause fatal acidosis and renal failure. This is due to the fact ethylene glycol is metabolised hepatically by alcohol dehyrogenase to gylcoaldehde which is subsequently oxidised to glycolic acid, glyoxylic acid and finally oxalic acid.
Treatment
Traditionally ethanol therapy has been used as treatment as at a high concentration, this absorbs alcohol dehdrogenese preventing it from metabolising the ethylene glycol and allowing it to be released by excretion in its original form by the kidneys. However due to the toxicity of ethanol, an alternative therapy using fomepizole, or 4-methylpyrazole is used.[7]
Other Harmful Effects
- May cause irritation to the eyes and respiratory tract
- Short term exposure may cause dizziness, spontaneous eye movement, increased heart rate, high blood pressure, nausea, vomiting and agitation.
- Skin exposure in the long term may cause dermatitis
- Severe intoxication can cause damage to the central nervous system.
References
- ↑ R. Landau and B.J Ozero Ethylene Glycol Commercial History
- ↑ H.Bock and G.Wozny Analysis of Distillation and Reaction Rate in Reactive Distillation
- ↑ J.D Pritchard Ethylene Glycol Production and Uses Retrieved on 1 December 2007
- ↑ Charles B. Jordan U.S Army Antifreeze after 50 years
- ↑ PET synthesis Retrieved on the 25th November
- ↑ J.D Pritchard Ethylene Glycol Production and Uses Retrieved on 1 December 2007
- ↑ Antizol Ethylene Glycol Poisoning Retrieved on the 25th November 2007
