It07:Erythromycin
| Erythromycin | |||
|---|---|---|---|
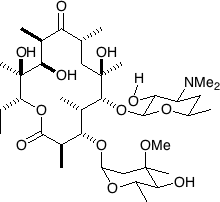 | |||
| IUPAC Systematic name | |||
| 6-(4-dimethylamino-3-hydroxy-6-methyl-oxan-2-yl)oxy-14-ethyl-7,12,13-trihydroxy-
4-(5-hydroxy-4-methoxy-4,6-dimethyl-oxan-2-yl)oxy-3,5,7,9,11,13-hexamethyl- 1-oxacyclotetradecane-2,10-dione | |||
| Other Names | |||
| E-Base, E.E.S., E-Mycin, ERYC, EryPed, Ery-Tab, Erythrocin, Illosone, PCE DisPErtab, Robimycin, Robitabs, WyamycinS | |||
| Indentifiers | |||
| ATC Code | J01 FA01 | ||
| CAS number | 114-07-8 | ||
| PubChem (CID) | [1] | ||
| SMILES | O=C([C@H(C)[C@@H](O)[C@](C)(O)[C@@H]
(CC)OC1=O)[C@H](C)C[C@]([C@H](OC2C(O[H]) C(N(C)C)CC(C)O2)[C@@H](C)[C@H] OC3OC(C)C(O)C(C)(OC)C3)[C@H]1C)(O)C ] | ||
| Chemical Data | |||
| Molecular formula | C37H67NO13 | ||
| Molar mass | 733.94 g/mol | ||
| Pharmacokinetic Data | |||
| Bioavailability | 100% | ||
| Protein Binding | 90% | ||
| Metabolism | Hepatic | ||
| Half life | 1.5 hours | ||
| Excretion | Bile | ||
| Therapeutic considerations | |||
| Pregnancy cat. | A | ||
| Legal status | Prescription only | ||
| Routes | Oral, Intramuscular, Intravenous. | ||
Erythromycin is an antibiotic effective against gram-positive cocci (such as Streppococcus aureus) and certain gram-negative organisms (such as (eg B.pertussis) furthermore, erythromycin can be used against certain mycoplasmic infections (e.g. Chlamydia). It is commonly used in the treatment of throat infections and pneumonia, especially when the patient is allergic to penicillin. It belongs to the group known as macrolide antibiotics, due to the 14 membered lactone (cyclic ester) ring from which its functionality derives.
Discovery
Erythromycin was discovered in 1949 by the research group of J.M. McGuire, whilst working for the Indianapolis based pharmaceutical giants Eli Lilly and Company. The drug was found in soil samples from the Iloilo province of the Phillipines, and was discovered to be a metabolic product of the fungus Saccharopolyspora erythraea (formerly known as Streptomyces erythraeus)[1]. Erythromycin was the first macrolide antibiotic, a group of great importance in modern medicine – five macrolides are in current medical usage[2] It was first patented in 1952 as Ilosone® in reference to the location of its origin.
Action
Erythromycin inhibits bacterial protein synthesis by binding to the 23S rRNA molecule within the 50S ribosome sub-unit, thus blocking the continued growth of the polypeptide chain. Furthermore, erythromycin also inhibits the formation of new ribosomal sub-units, reducing the number of functional ribosomes in the bacterium and hindering protein synthesis. Obviously, this prevents the bacteria growing and ends the infection[3]
Side-Effects
Compared to other common antibiotics such as penicillin, the side-effects of erythromycin are relatively common and unpleasant. It is for this reason that erythromycin is not commonly used as a front line antibiotic. Frequent effects include abdominal pain, diarrhoea and vomiting. Other less common effects include dizziness, confusion, and vomiting. Other, less common effects reported include: confusion, venous irritation and thrombophlebitis.
Dosage
As erythromycin has a half-life of just 1.5 hours, it is generally required to take the medication four times daily. A standard dosage would be 500mg taken four times a day.[4]
Spectra


References
- ↑ {http://ccc.chem.pitt.edu/wipf/Frontiers/Josh_Frontiers.pdf}
- ↑ {http://www.lareb.nl/documents/kwb_2002_4_macro.pdf}
- ↑ {http://aac.asm.org/cgi/reprint/45/1/1}
- ↑ {http://www.nhstaysideadtc.scot.nhs.uk/TAPG%20html/Section%2014/drugdosages.htm}
- ↑ J. Pharm. Sci. Vol. 86, No. 11, 1243{http://download.interscience.wiley.com/cgi-bin/fulltext?ID=72505290&PLACEBO=IE.pdf&mode=pdf}
- ↑ J. Pharm. Sci. 95, 8, 1723-32{http://www3.interscience.wiley.com/cgi-bin/fulltext/112657014/HTMLSTART}
- ↑ J. Agric. Food Chem., 53 (25), 9689 -9694,{http://pubs.acs.org/cgi-bin/abstract.cgi/jafcau/2005/53/i25/abs/jf0520894.html}
