It07:Epinephrine
| Epinephrine | |
|---|---|

| |
| IUPAC Systematic name | |
| (R)-4-(1-hydroxy-2-(methylamino)ethyl)benzene-1,2-diol | |
| Other name | |
| Adrenaline | |
| Identifiers | |
| ATC Code | A01AD01, B02BC09, C01CA24, R01AA14, R03AA01, S01EA01 |
| CAS number | 51-43-4, 150-05-0, 329-65-7, 6912-68-1 |
| PubChem (CID) | 838 |
| SMILES | OC1=C(O)C=CC((C@H)(CNC)O)=C1 |
| Chemical Data | |
| Molecular formula | C9H13NO3 |
| Molar mass | 183.204 g/mol |
| Physical Data | |
| Melting Point | 212°C |
| Electrical Moment | Dipole Moment |
| Optical Rotation | α 50.49° [1] |
| Pharmacokinetic Data | |
| Bioavailability | Nil |
| Protein Binding | {{{Protein_binding}}} |
| Metabolism | Adrenegenic Synapse |
| Half life | 2 minutes |
| Excretion | n/a |
| Therapeutic considerations | |
| Pregnancy cat. | C (US) |
| Legal status | Prescription Only |
| Routes | Intravenous, Intramuscular, Endotracheal |
Introduction
Epinephrine (or adrenaline) is a stress hormone; part of the catecholamine family; that induces the “fight or flight” response whereby the heart rate, breathing and pupil diameter all increase. Blood flow is also diverted from the digestive tract and skin to the legs for example, in preparation for the “fight or flight” response. It is released from the central part of the adrenal gland known as the adrenal medulla. Adrenaline targets various cells and in particular the α1, α2, β1, β2 and β3 adrenergic receptors.
The molecules 3D structure can be explored below.
Ephinephrine |
β adrenergic receptors
A member of the serpentine receptors, these receptors are located on muscle, adipose tissue and liver cells. Once binding of the hormone occurs there is a seven step process (similar mechanism for all 3 types of β receptors) that results in the formation of cAMP. This stimulates PKA phosphorylation of cellular proteins which in turn causes effects such as greater lipolysis, bronchiole dilation, heart rate and insulin release.
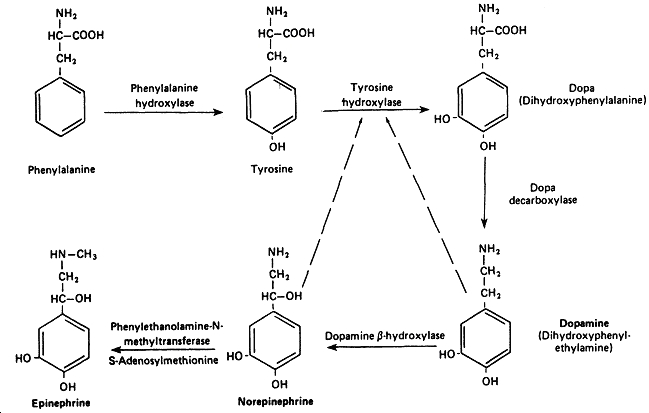
Biosynthesis
Epinephrine is a product in the synthesis of catecholamines from the amino acid tyrosine. The key step in this reaction sequence is that of Dopa to Dopamine. This is because low levels of dopamine as this causes schizophrenia, whilst at high levels
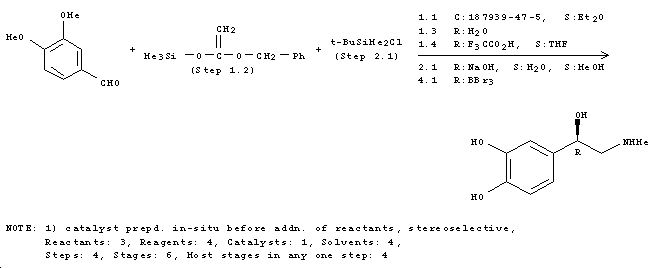
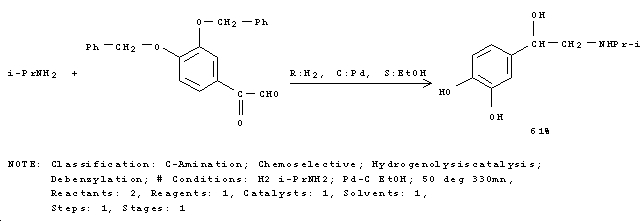
Commercial Synthesis
Due to the effects of epinephrine, it is used in the treatment of cardiac arrests as it induces ventricular relaxation via β1 and β2 adrenergic receptors[3]. Hence synthetic routes such as the following are of importance beyond biosynthesis. This reaction is an example of synthesis of epinephrine from readily available reagents[4]. The enantiomer specific reaction yield of 47% is obtained in reasonable time and reaction conditions.
Synthetic Analogs of Epinephrine
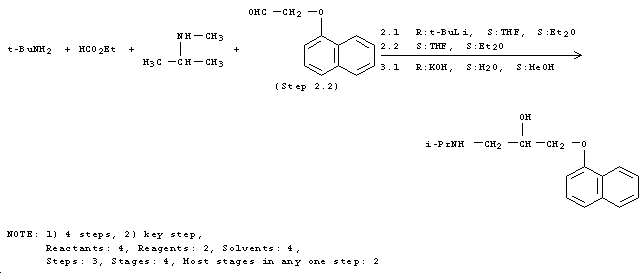
Agonists such as isoproterenol (synthetic route below[5]) imitate the effect of epinephrine with similar (or even slightly better) affinity.
Antagonists such as propranolol (synthetic route below[6]) merely block the effect from occurring by obstructing the receptor site with far superior affinity than epinphrine.
These synthetic analogs provide ways in which to counter the negative effects that epinephrine can induce such as platelet aggregation[7].
IR Condensed Phase Spectrum[8]
(-)-Adrenaline hydrogen (+)-tartrate Crystal Structure
C9H14NO3+, C4H5O6-
Epinephrine |
Commercial Availability
Adrenaline is not easily commercially available, although through prescription it is available for a patient to self treat anaphylactic shock; essentially an allergic reaction to for example, peanuts. Commercially marketed as the "Epi-Pen", the dosage is contained in a spring loaded needle that is jabbed firmly into the thigh for use.
References
- ↑ Abderhalden; Guggenheim; HSZPAZ; Hoppe-Seyler's Z. Physiol. Chem.; 57; 1908; 329
- ↑ http://rfumsphysiology.pbwiki.com/f/nt%202.bmp
- ↑ A. Kaumann, S. Bartel, P. Molenaar, L. Sanders, K. Burrell, D. Vetter, P. Hempel, P. Karczewski and E. G. Krause, Circulation, 1999, 99, 65-72.
- ↑ R. A. R. Singer, Tetrahedron Letters, 1997, 38, 927.
- ↑ G. G. Fodor, Journal of the American Chemical Society, 1949, 71, 1045.
- ↑ A. A. Solladie-Cavallo, Tetrahedron Letters, 1990, 31, 2157.
- ↑ R. E. Raulli, B. Jackson, N. Tandon, M. Mattson, K. Rice and G. A. Jamieson, Biochimica Et Biophysica Acta-Molecular Cell Research, 1994, 1224, 175-180.
- ↑ http://webbook.nist.gov/cgi/cbook.cgi?ID=C51434&Units=SI&Mask=80#IR-Spec