It07:EDTA
| It07:EDTA | |
|---|---|
| |
| General | |
| IUPAC Systematic name | EDTA |
| Other names | Diaminoethanetetraacetic acid |
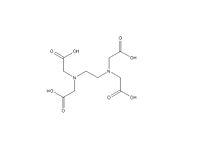
| Molecular formula | C10H16 N2 O8 |
| SMILES | OC(CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O)=O |
| CAS number | 60-00-4 |
| Molar mass | 292.25 gmol-1 |
| Properties | |
| Density | 0.86gcm3 |
| Melting point | 237-245 °C (280-284 °F) |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox disclaimer and references | |
Overview
EDTA is a widely-used chemical compound with the chemical formula (HO2CCH2)2NCH2CH2N(CH2CO2H)2. The molecule is able to bind to metals by both the two amine groups and the four carboxylate, forming 6 coordinate complexes.
|
Cyclopentasiloxane |
Synthesis
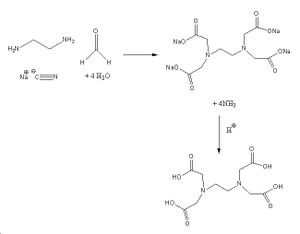
EDTA is normally synthesised from ethylenediamine, formaldehyde, sodium cyanide and water. This forms a sodium salt and acidic work up gives the desired product. One of the main problems with this reaction is the fact that the ammonia produced can undergo a side reaction with the sodium cyanide and formaldehyde to produce mono, di and triglycine. This can be solved by slow addituion of reactants, lower temperature and higher pH (to drive off the ammonia more quickly)[1]. Another problem is that ethylenediamine may not may not go to the fully substituted product. However the use of excess sodium cyanide and formaldehyde will solve this.
Safety
EDTA salts can start to decompose at temperatures above 204 oC[2]. Contact with metals such as aluminium, zinc or copper should be avoided as this can produce flammable hydrogen gas. Although non-flammable, under combustion EDTA can produce toxic or irritant compounds such as carbon monoxide, ammonia and various nitrogen oxides. Appart from this, good ventilation and general common sense (e.g. safety goggles, gloves etc.) safety should be used.
Spectra
Selected Uses
• In food, to act as a preservative, preventing the catalytic oxidation be metal ions, as a stabilizer or for iron fortification.
• In cosmetics, to improve the product stability.
• In oil production, to inhibit mineral precipitation within boreholes.
• In the beverage industry, to help to clean stains from bottles.
• In fuel gas cleaning, to remove the nitrogen oxide.
• In soft drinks, to mitigate the formation of benzene from the sodium benzoate and ascorbic acid present.
• In recycling, to aid in the recovery of lead from batteries.
Medicinal uses
• In chelation therapy, for the treatment of acute mercury and lead poisoning.
• In the evaluation of kidney functionality.
• As anticoagulant in blood samples.
Environmental Presence
Studies have shown the presence of EDTA in rivers(up to 100μg/L), drinking water(up to 40μg/L) and sewage treatment plants (up to 300μg/L)[3].However this is approximately one thousandth of the level needed to cause harm to any aquatic life [4]. EDTA does not biodegrade readily and as such is not removed by conventional water treatment. The exact cause of its stability is presently unknown [5] , however an adjustment of the pH and of the sludge residence time can cause it to be almost completely degraded. There are also numerous micro-organisms[6] that have been discovered to be able to degrade EDTA.
References
- ↑ Smith et al, J.Org.Chem, 14, 1949, 355-361 DOI:10.1021/jo01155a003
- ↑ http://www.dow.com/productsafety/finder/edta.htm#EnvironmentalInfo
- ↑ Sykora et al, Wat. Res., 35, 2001, 2010-2016 DOI:10.1016/S0043-1354(00)00455-3
- ↑ http://www.dow.com/productsafety/finder/edta.htm#EnvironmentalInfo
- ↑ Sykora et al, Wat. Res., 35, 2001, 2010-2016 DOI:10.1016/S0043-1354(00)00455-3
- ↑ Satroutdinov et al, Environ. Sci. Technol., 34, 2000, 1715-1720DOI:10.1021/es981081q
