It07:DIBAL
DIBAL Di-IsoButyl Aluminium Hydride
DIBAL |
| It07:DIBAL | ||||
|---|---|---|---|---|
| ||||
| General | ||||
| Systematic name | Di-Isobutyl Aluminium Hydride | |||
| Other names | DIBAL, DIBAL-H, DIBAH, DIBALH | |||
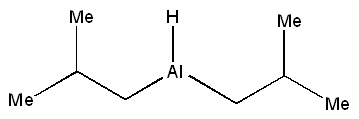
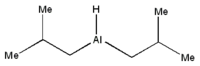
| Molecular formula | [(CH3)2CHCH2]2AlH | |||
| SMILES | [H][Al]CC(C)C.CCC(C)C | |||
| Molar mass | 142.22 | |||
| Appearance | Clear, colourless liquid | |||
| CAS number | 1191-15-7 | |||
| Properties | ||||
| Density & phase | 0.798 g/cm³ | |||
| Melting point | 255.15 K | |||
| Boiling point | 389.15-391.15 K | |||
| Hazards | ||||
| MSDS | External MSDS | |||
| Main hazards | Corrosive, highly flammable. | |||
| Thermodynamic data |
Liquid | |||
| 3-D Structure | ||||
| 3D |
| |||
Introduction
[1]
DIBAL has rapidly established itself as a reducing agent of choice in organic synthesis. It is most effective at reducing electron-rich carbonyl groups and has largely replaced the use of metal hydrides (including LiAlH4 and NaBH4) due to economic advantages. It demonstrates increased selectivity and a considerably lower cost per reducing equivalent. It is also a particularly easy-handling material as it is a liquid that is miscible in many solvents.
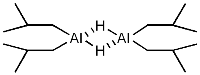
DIBAL exists as a dimer due to increased stabilisation:
Reduction of Esters to Aldehydes using DIBAL
Chemists often come across a problem when reducing an ester to an aldehyde. This is because the aldehyde product is more susceptible to reduction than the starting ester and therefore the reduction process continues to the next oxidation level – to the alcohol. DIBAL, however, can be used as a reagent that will carry out this reduction in 1 step and stops at the aldehyde without further reduction.
At -70oC the tetrahedral intermediate is stable. It collapses to the aldehyde on aqueous work-up (slow quenching with methanol followed by full quenching with water), but by this stage excess DIBAL has been destroyed so as to prevent any further reduction.
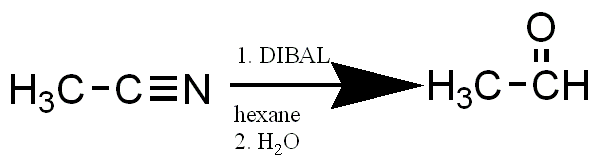
Reduction of Nitriles to Aldehydes using DIBAL
Mechanism:
i. The first step of this mechanism involves an acid-base reaction between a lone pair of electrons on N with the AL in DIBAL.
ii. The second step involves the transfer of a hydride ion from DIBAL to the C of the nitrile group.
iii. The final step is the hydrolysis of the aluminium complex which results in the formation of the desired aldehyde.
Safety Information
Neat DIBAL is pyrophoric (capable of igniting spontaneously in air), and its solutions react violently with water, oxygen and other related compounds. It should always be handled with care in a fume hood.
Potential Health Effects
- Eye: Causes eye burns.
- Skin: Causes skin burns.
- Ingestion: Causes gastrointestinal tract burns.
- Inhalation: Harmful if inhaled. Causes chemical burns to the respiratory tract.
NOTE: Only use dry chemical to extinguish DIBAL-induced fires. DO NOT USE WATER.
References
- ↑ http://www.akzonobel-polymerchemicals.com/NR/rdonlyres/CB29DA1C-1144-47F4-888A-B20FC61E17F5/11037/Diisobutylaluminumhydride_ma_row_eng_tb.pdf
- ↑ “ORGANIC CHEMISTRY” Clayden, Greeves, Warren & Wothers
- ↑ http://www.cliffsnotes.com/WileyCDA/CliffsReviewTopic/Synthesis-of-Aldehydes.topicArticleId-23297,articleId-23278.html
- ↑ http://www.sigmaaldrich.com/catalog/search/ProductDetail/ALDRICH/256811