It07:Cyanidin
| It07:Cyanidin | |
|---|---|

| |
| General | |
| Systematic name | 2-(3,4-dihydroxyphenyl)chromene-3,5,7-triol |
| Other names | flavan-3-ol, cyanidol [1] |
| Molecular formula | C15H10O6 |
| SMILES | O=C(C(O)=C3)C=C/C3=C2C(O)=CC1=C(O)C=C(O)C=C1O/2 |
| Molar mass | 286 |
| Appearance | red in acidic solution, blue in alkali solution, violet in neutral solution |
| CAS number | 528-58-5 [2] |
| Supplementary data page | |
| Structure and properties |
n, εr, etc. |
| Thermodynamic data |
Phase behaviour Solid, liquid, gas |
| Spectral data |  UV Spectrum (Cy-cyanidin) [3] UV Spectrum (Cy-cyanidin) [3]
|
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox disclaimer and references | |
|
Pentahelicene |
Introduction
Cyanidin, a type of anthocyanin, is an organic compound belonging to a large group of phenolic compounds called flavonoids and is one of the pigments responsible for the red colour in many red berries, including cherries, raspberries and cranberries. It is in its greateast concentration in the skin of the fruit, and is the most widespread anthocyadinin in the plant kingdom. Of over 150 different flavonoids, anthocyanins show the greatest capacity as antioxidants, which help reduce the risk of cancer and heart disease, and their anti-inflammatory properties are considered as successful as ibuprofen and aspirin. Anthcyanins are thought to be likely to play a vital role in future cancer treatment and therapy. They have also shown to relieve pain from arthritis and gout and may help in preventing obesity and diabetes.
Structure
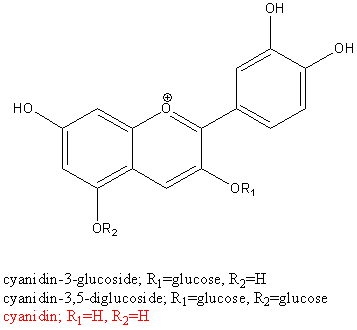
Anthocyanins are split into anthocyanin glucosides, and their corresponding sugar free aglycones, called anthocyanidins. Cyanidin is the aglycon of the most common, naturally occurring anthocyanins, cyanidin-3-glucoside and cyanidin -3,5-cyanidin (3 structures shown below).
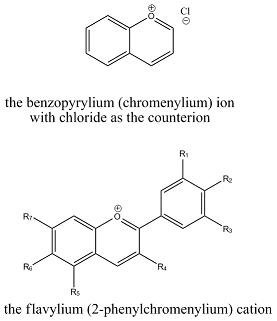
The reason for the colour or cyanidin and other anthocyadinins is the benzopyrylium (chromomenylium) ion, a polymethine dye. More specifically, they are salt derivatives of the flavylium cation, which forms the scaffolding of all anthocyadinins. The positive charge on the oxygen atom makes the anthocyanins different from other flavonoids.
(In the picture below, R1=OH, R2=OH, R3=H, R4=OH, R5=OH, R6=H and R7=OH for cyanidin.)
Properties
The colour of cyandin is pH sensitive, appearing red in acidic conditions, violet in neutral and blue in alkali conditions. Anthocyanins are responsible for the red colour in leaves during autumn, though the soil acidity affects the colour seen.
Synthesis
Cyanidin and other anthocyanidins are derived from flavonoids, the biosynthesis of which has been widely researched. However, the mechanism for the synthesis of anthocyanidins and cyanidin from the flavonoids is still not entirely understood.
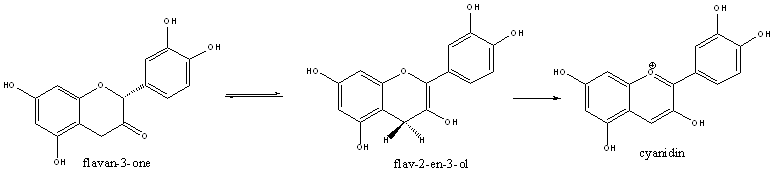
All flavanoids are derived from flavanones, and in the case of cyanidin, flavan-3-one. One considered route is that the flavan-3-one is converted to flav-2-en-3-ol by hydroxylation, which then spontaneously oxidises to cyanidin in the presence of oxygen.
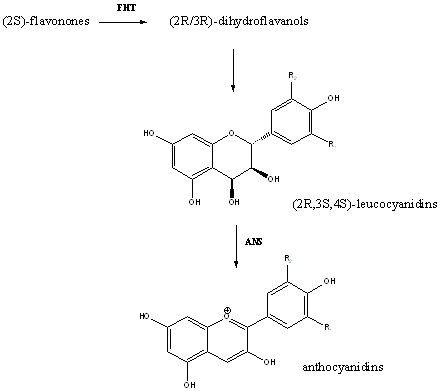
An alternative route for the synthesis of anthocyanidins is the conversion of (2S)-flavonones to (2R/3R)-dihydroflavanols by hydroxylation, and then the reduction of this to (2R,3S,4S)-leucoanthocyanidins by dihydroflavonol 4-reductase (DFR). The leucoanthocyanidins then become anthocyanidins with the action of the enzyme, ANS.
Activity
Antioxidant Activity
Cyanidin has shown to have higher antioxidant capacity than its corresponding anthocyanins. Hydroxyl (OH-) and peroxyl (ROO-) radicals are reactive oxygen species produced in living species through metabolic reactions. Enzymes and antioxidant compounds in the body protect it from the oxidative damage caused by these oxygen species. However, free radical production can exceed the antioxidant capacity and so they can then go on to attack lipids, proteins and DNA causing various diseases. The mechanisms for the antioxidant acitivty of cyanidin is still not fully understood. However, it is thought they react with the free radicals and so preventing the formation of new reactive free radicals or they chelate (form complexes with) metal ions such as Fe2+, which catalyse lipid peroxidation. It is believed the reason cyanidin is more efficient at antioxidant activity than anthocyanins is due to the lack of the sugar residues in the C3 position. The more the sugar residues there are, the lower the activity. The reason for this, however, is still unknown. The stability of the resulting aryloxyl radical, following the reaction of cyanidin and the oxygen radicals, also plays an important role in its antioxidant activity. If the resulting radical is very stable, it will not then react further and so the unpaired electron will have been effectively ‘removed’ from the system. The –OH groups on the B ring at carbons 3’ and 4’ help stabilise the radical produced.
Anti-Inflammatory Activity
Unlike anthocyanins, cyanidin showed to inhibit the activity of the cyclooxygenase enzymes PGHS-1 and PGHS-2, which appear to cause pain and inflammation of the cells. It is though that anthocyanins are unable to inhibit these enzymes because at high concentrations, they act as oxygen carries and can, therefore, increase the uptake of oxygen.
References
- ↑ Phytochemicals - Cyanidin - http://www.phytochemicals.info/phytochemicals/cyanidin.php
- ↑ Cyanidin - http://www.chemindustry.com/chemicals/1658829.html
- ↑ J. Agric. Food Chem., 47 (3), 1083 -1091, 1999. 10.1021/jf9809582 S0021-8561(98)00958-3, T. Miyazawa, K. Nakagawa, M. Kudo, K. Muraishi, and K. Someya
- ↑ 4.0 4.1 Cyanidin - http://en.wikipedia.org/wiki/Cyanidin
- ↑ 5.0 5.1 Montmorency Cherry Nutrition Report - http://www.cherryactive.co.uk/img/Montmorency%20Cherry%20Nutrition%20Report.pdf
- ↑ Anthocyanins - http://en.wikipedia.org/wiki/Anthocyanins
- ↑ Cyanidin - An Anthocyanin - http://chemistry.about.com/library/graphics/blancy.htm
- ↑ Anthocyanidin synthase from Gerbera hybrida catalyzes the conversion of (+)-catechin to cyanidin and a novel procyanidin by Frank Wellmann, Markus Griesser, Wilfried Schwab, Stefan Martens, Wolfgang Eisenreich, Ulrich Matern and Richard Lukačin - http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6T36-4J90V1R-6&_user=217827&_coverDate=03%2F06%2F2006&_rdoc=1&_fmt=&_orig=search&_sort=d&view=c&_acct=C000011279&_version=1&_urlVersion=0&_userid=217827&md5=57a3e2edff4dcf4958d114256d7f34a0
- ↑ 9.0 9.1 J. Nat. Prod. 1999, 62, 294-296 - Antioxidant and Antiinflammatory Activities of Anthocyanins and TheirAglycon, Cyanidin, from Tart Cherries by Haibo Wang, Muraleedharan G. Nair, Gale M. Strasburg, Yu-Chen Chang, Alden M. Booren, J. Ian Gray, and David L. DeWitt