It07:Cyameluric Acid
| It07:Cyameluric Acid | |
|---|---|
| General | |
| Systematic name | 1,3,4,6,7,9,9b-heptaaza-phenalene-2,5,8-triol |
| Other names | 1(9)H,3(4)H,6(7)H-1,3,4,6,7,9,9b-heptaaza-phenalene-2,5,8-trione |
| Molecular formula | C6H3N7O3 |
| SMILES | OC3=NC1=[N]2C(N=C(O)N=C2=N3)=NC(O)=N1 |
| Molar mass | 221.13 |
| CAS number | 1502-46-1 |
| Properties | |
| Density & phase | 0.935g/cm³ |
Structure of Cyameluric Acid
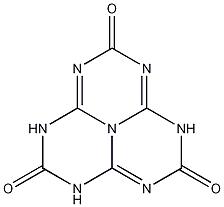 [1] There are in fact 17 tautomeric forms in total of this substance. The structure shown is the most stable tautomer (the Trioxo 2,5,8 form). Cyameluricacid is commonly drawn as its less stable tautomer
[1] There are in fact 17 tautomeric forms in total of this substance. The structure shown is the most stable tautomer (the Trioxo 2,5,8 form). Cyameluricacid is commonly drawn as its less stable tautomer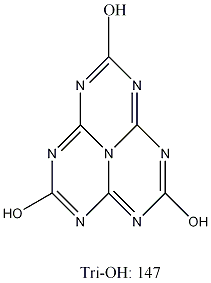
3D Model of Cyameluric Acid
Sibutramine |
Synthesis
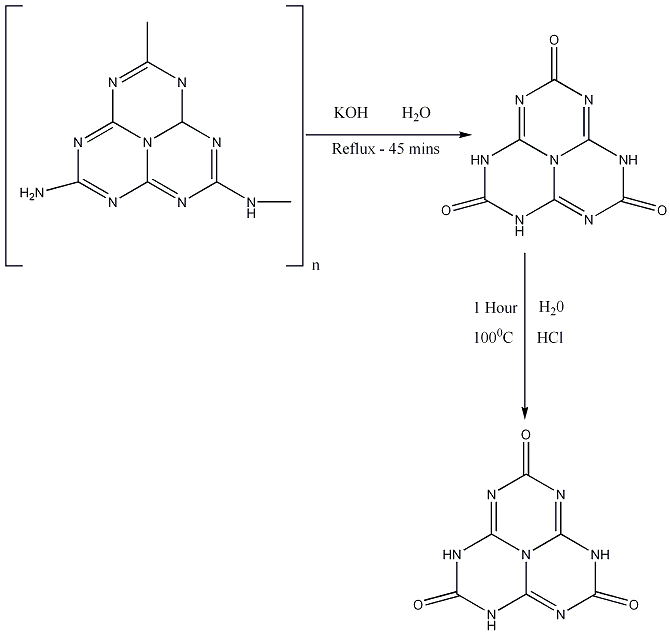
The following diagram shows a possible synthesis for the the Trioxo 2,5,8 tautomer of cyameluric acid4:
History and Origin
A derivative of Cyameluric acid was the last molecule to be worked on by Linus Pauling before he died death in 1994. The structure of a Cyameluric acid with a hydroxy group replaced by an azide was found on his chalkboard after his death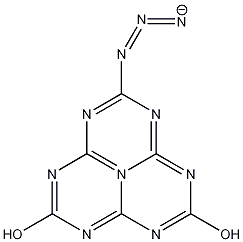 [2]
[2]
Pauling et al first postulated the existence of a cyamleuric nucleas in 1937. In 1940 Redeman and Lucas made several attempts to prepare cyameluric esters and amides and in the 1960's and 1970's cyameluric salts and other s-heptazine derivatives such as melem (C6N7(NH2)3) were investigated by Finkelstein.3
Linus Pauling
Linus Pauling originated from Portland, Oregon, USA, remaining there to complete a BSC in Chemical Engineering in 1922. He received his PhD in Chemistry in 1925 and was appointed Teaching Fellow in Chemistry at California Institute of Technology. Over his long career, Pauling's interests have been many and varied. Early on he was interested in molecular spatial configurations, winning the Nobel Prize for chemistry in 1954 for "his research into the nature of the chemical bond and its application to the elucidation of the structure of complex substances"<ref>Linus Pauling Nobel Prize Biography[1]<ref>. This interest developed into biological chemistry, where he began looking at proteins and various molecules comprised of proteins. During the second world war Pauling's attention turned to the protection of the country, acting as consultant to many national security commissions, working on rocket propellents, explosives and other war-related items. Following the dropping of the atomic bomb, Pauling became an anti-war activist, sitting on Albert Einstein's Committee of Atomic Scientists, going on to win the Nobel Peace Prize in 1963 after helping to get nuclear testing banned.
Uses
Cyameluric acid can be used as an adsorbent for the analysis of certain gas-chromotography analyses. Interests arose in cyameluric acid and its related structures because of its potential use as building blocks for graphitic carbon nitrides. Cohen predicted that C3N4 materials may exist that are harder than diamond.
Another potential use is that of burn rate supressants for solid rock propellants and flame retardernts. This use is due to the very high thermal stablity of the C6N7 nucleas (~500°C). Also the UV, luminescence and NLO properties may be used in a fashion to develop photo stabilisers and sensitisers.3
References
- ↑ E Horvath-Bordon et Al, Alkalicyamelurates, M3[C6N7O3].H2O, M=Li, K, Na, Rb, Cs: UV-Luminescent and thermally very stable ionic tri-s-triazine derivatives http://pubs.rsc.org/ej/DT/2004/b412517g/
- ↑ Theoretical study of cyameluric acid and related compounds http://www.arkat-usa.org/?VIEW=MANUSCRIPT&MSID=917
3. The Tautomeric Forms of Cyameluric Acid Derivatives 4. Alkalicyamelurates, M3[C6N7O3]xH2O, M = Li, Na, K, Rb, Cs: UV-luminescent and thermally very stable ionic tri-s-triazine derivatives.