It07:Copper arsenate
| It07:Copper arsenate | |
|---|---|
| |
| General | |
| Systematic name | Arsenic acid (H3AsO4) copper(2+) salt (2:3) |
| Other names | Copper arsenate, Copper orthoarsenate, Tricopper arsenate, Tricopper orthoarsenate |
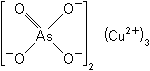
| Molecular formula | As2Cu3O8 |
| Molar mass | 468.476 g/mol |
| Appearance | Pale green solid |
| CAS number | 7778-41-8 |
| Properties | |
| Density & phase | 5.07 - 5.25 g/ml |
| Solubility | Highly soluble in HCl |
| Structure | |
| Coordination geometry |
{{{Coordination}}} |
| Crystal structure | {{{Crystal_Structure}}} |
| Dipole moment | {{{DM}}} D |
| Hazards | |
| MSDS | External MSDS |
| Main hazards highly toxic, carcinogenic | |
| NFPA 704 | {{{NFPA}}} |
| Supplementary data page | |
| Related compounds | |
| Other anions | {{{Other_anion}}} |
| Other cations | {{{Ohter_cation}}} |
| Related compounds | {{{Relative_Compounds}}} |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox disclaimer and references | |
| Structure | |
|---|---|
| Molecular shape | {{{MolShape}}} |
| Coordination geometry |
{{{Coordination}}} |
| Crystal structure | {{{CrystalStruct}}} |
| Dipole moment | {{{Dipole}}} D |
Scheele's Green
 Taken from http://de.wikipedia.org/wiki/Carl_Wilhelm_Scheele
Taken from http://de.wikipedia.org/wiki/Carl_Wilhelm_Scheele
Developed in 1775, by the Swedish-German chemist Carl Wilhelm Scheele, Scheele's green (also known as Schloss green- Schloss meaning castle/mansion in German) is a vibrant yellowish green colour, composed of a mixture of various copper arsenite compounds. It is the predecessor to the more vibrant Paris Green (also known as Schweinfurt Green, or Emerald Green) and was used as an insecticide, as well as a pigment, until the hazards connected to its use were acknowledged. Before the discovery of this pigment, the known green pigments were based on copper carbonate and had the tendency to fade, which made this choice of colour unsustainable. The popularity of Scheele's green was based on its bright colour, as well as the fact that it was a lot more durable than previous pigments, which meant that the colour green could be used in its full splendour, without having to worry too much about its progressive degradation.

3-D image of CuAsHO3
Pentahelicene |
Preparation [1]
The pigment was originally prepared by making a solution of sodium carbonate at a temperature of around 90°C, then slowly adding in arsenious oxide, while constantly stirring until everything had dissolved. This sodium arsenite solution was then added to a copper sulphate solution. The sodium would displace the copper, which resulted in the formation of the desired product in the form of a green precipitate. This copper arsenite was then filtered off and warmed to around 43°C to dry the crystals. To enhance the colour, the salt was subsequently heated to 60-70°C. The intensity of the colour depends on the copper : arsenic ratio, which in turn was affected by ratio of the starting materials, as well as the temperature.
Another method used to produce Scheele's green was by directly adding dilute copper sulphate solution to an alkali solution of arsenic. This would then precipitate- the precipitate also being Scheele's green.
It has been found that Scheele's green was composed of a variety of different compounds, including copper metaarsenite (CuO.As2O3), copper arsenite salt (CuAsHO3 and Cu3(AsO3)2.3H2O)), neutral copper orthoarsenite (3CuO.As2O3.2H2O), copper arsenate (CuAsO2 and Cu(AsO2)2), copper diarsenite (2CuO.As2O3.2H2O).
Uses
The beautiful green colour of this compound meant that it was largely used in pigments- be these for paints, dyes for cloths, or otherwise. It was even used to colour foodstuffs, such as sweets and alcoholic beverages, to colour candles and to paint toys. Later, once its toxicity was vaguely understood, Scheele’s green was used as an insecticide. Today it is no longer used in any form.
Toxicity
Today it is intuitive to most that an complex containing arsenic is most likely to be toxic, however in the 19th century this was not really known. 19th century journals reported of children wasting away in bright green rooms, of ladies in green dresses swooning and newspaper printers being overcome by arsenic vapours. There is even one report of a Christmas party that resulted in mass arsenic poisoning, due to the use of green candles. [2]
Arsenic poisoning was usually caused by the inhalation of arsine gas (AsH3), which can be released from compounds containing arsenic following certain chemical processes, such as heating, or its metabolism by an orgnanism. Fungi such as Scopulariopsis, or the Paecilomyces species release arsine gas, when they are growing on a substance containing arsenic.[3] [4] In these compounds, the Arsenic is either pentavalent, or trivalent (arsenic is in group 15), depending on the compound. In humans, arsenic of these valences is readily absorbed by the gastrointestinal tract, which accounts for its high toxicity. Pentavalent arsenic tends to be oxidised to trivalent arsenic and trivalent arsenic tends to proceed via oxidative methylation in which the trivalent arsenic is made into a mono, di and trimethylated products by the S adenosyl methionine (an enzyme) and its cofactor glutatione.[6][7]. Arsenic is not only toxic, but it also has carcinogenic effects [7].
Scheele's Green and Napoleon
During Napoleon's exile in St. Helena, he resided in a very luxurious bright green room. His cause of death is generally believed to be stomach cancer, however, analysis of his hair samples revealed significant amounts of arsenic. As St. Helena has a rather damp climate, it is more than possible that fungus grew on the wall. So as to remove the toxic arsenic, the fungus would release it in the form of arsine gas, which when inhaled causes arsenic poisoning.[7]
References
[3] Fungus Glossary
[4] Fungus Glossary
[5] http://www.grand-illusions.com/napoleon/images/walpaper.JPG
[6] The enigma of arsenic carcinogenesis: role of metabolism
