It07:Coniine
| It07:Coniine | |
|---|---|
| |
| General | |
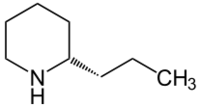
| Systematic name | (2S)-2-propylpiperidine |
| Other names | Coniine , Piperidine, 2-propyl-, (2S)- , (+)-Coniine, (S)-(+)-Coniine, (S)-2-Propylpiperidine, (S)-Coniine,Cicutin, Cicutine, Conicine, Coniin, Koniin, Piperidine, 2-propyl-, (S)- |
| Molecular formula | C8 H17N |
| SMILES | N1C(CCC)CCCC1 |
| Molar mass | 127.23 g/mol |
| CAS number | 3238-60-6 |
| Properties | |
| Density & phase | 0.812 g/cm³ |
| Boiling point | 166.2 oC at 760 mmHg |
| Hazards | |
| Flash point | 48.4 oC |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox disclaimer and references | |
Coniine is a member of the alkaloid family. It is found in poison hemlock plants and it was first synethsized in 1886. Coniine is famously associated with Socrates who was forced to drink it when he was condemned to death in 399 B.C. Coniine is an example of a neurotoxin and can lead to paralysis and fatality.
3D Molecular Structure
Coniine |
History of Synthesis
Coniine was the first ever alkaloid to be synthesized. Coniine was synthesized completely in 1886 by Ladenburg and this was done by firstly heating at 300oC methylpyridinium leading to the formation of 2-methyl pyridine. The addition of acetalaldehyde and a lewis base then led to the formation of 2-propenylpryride which was then reduced with sodium in alchol to(+/-) coniine.

Bergmann in 1932 carried out an alternative synthesis. 2-methyl pyridine and phenyl lithium was reacted, forming an akly lithium compound. This was followed by a nucleophilic substitution reaction using bromoethane and then reduction using sodium in ethanol.
[1]
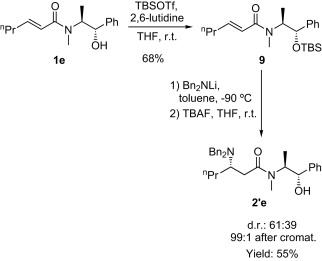
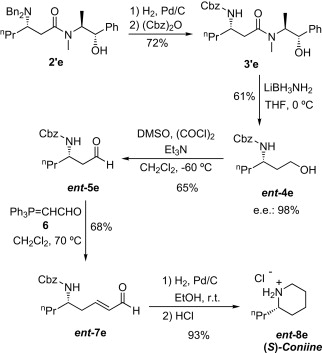
Asymmetric synthesis of (S)-coniine