It07:Cholesterol
Cholesterol
3D Structure
| It07:Cholesterol | |
|---|---|
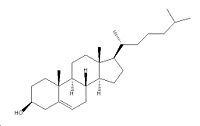
| |
| General | |
| Systematic name | 17-(1,5-dimethyl-hexyl)-10,13-dimethyl-2,3,4,7,8,9,10,11,12,13,14,
15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-3-ol |
| Other names | cholest-5-en-3(beta)-ol
cholesterin |
| Molecular formula | C27H46O |
| SMILES | O[C@H](C4)CC[C@@]1(C)C4=CC[C@]2([H])[C@@]([H])1 CC[C@@]3(C)[C@]([H])2CC[C@@H]3[C@H](C)CCCC(C)C |
| Molar mass | 386.65354g/mol [7] |
| Appearance | Soft, waxy yellow solid |
| CAS number | 57-88-5, 474-77-1, 14868-17-8, 23820-70-4, 34026-89-6, 57759-45-2, 71869-93-7, 121155-47-3, 138456-89-0 |
| ACX Number | X1001660-1 [8] |
| International Chemical Identifier (InChI) | InChI=1/C27H46O/c1-18(2)7-6-8-19(3)23-11-12-24-22-10-9-20-
17-21(28)13-15-26(20,4)25(22)14-16-27(23,24)5/h9,18-19,21 -25,28H,6-8,10-17H2,1-5H3/t19-,21+,22+,23-,24+,25+,26+,27-/m1/s1 |
| Properties | |
| Density & phase | 1.067g/cm³ [8] |
| Melting point | 148°C (421K) |
| Boiling point | 360°C (633K) [8] |
| Hazards | |
| MSDS | MSDS from Sigma Aldrich |
| Main hazards | None |
| Flash point | 250°C [8] |
Crystal structure of cholesterol
Cholesterol crystal |
Introduction
Cholesterol is a lipid with a waxy, fatty texture that doesn’t dissolve in water [9]. To be specific, cholesterol is a sub-set of lipids known as a steroid [9]. It can be found naturally in the bloodstream and tissue of animals (especially the liver, spinal cord and the brain) where it makes up cell membranes; is used to make vitamin D, adrenal gland hormones and sex hormones: and carries out many other functions [9]. It must be transported between cells by carriers known as lipoproteins because it cannot dissolve in the blood [10]. The soft, waxy texture of cholesterol means that too much cholesterol in the diet can result in hypercholesterolemia – the medical term for a build up of cholesterol in the bloodstream – which can lead to heart attack [10].
|
|---|
| [9] |
Types of cholesterol
There are 3 types of cholesterol:
- Cholesterol carried around in the blood by Low Density Lipoproteins (LDL) (also known as “bad” cholesterol) is the most common form of cholesterol in the blood. The body needs cholesterol to work properly and cells usually absorb it from the bloodstream as they need it. However, an excess of LDL cholesterol in the bloodstream can result in an accumulation of LDL cholesterol along with other substances on the walls of arteries. This can result in a condition called atherosclerosis in which the build up of cholesterol forms plaque, a tough deposit that narrows the arteries. Blood flow can be blocked if a clot (thrombus) forms near to this plaque. If this blockage is near to heart muscle, a heart attack results. If it is near to any part of the brain, it leads to a stroke. Hence, high levels of LDL increase the risk of heart disease [10].
- The 2nd type of cholesterol is a genetic variation of LDLs known as Lp(a) cholesterol. High levels of Lp(a) cholesterol are believed to increase the risk of heart disease by leading to atherosclerosis prematurely, although the exact reason why is not known. It is believed that Lp(a) cholesterol may react with substances contained in the lesions in the artery walls, resulting in an accumulation of fatty deposits [10].
- Cholesterol carried by High Density Lipoproteins (HDL) (also known as “good” cholesterol) accounts for approximately 25-33% of the body’s cholesterol. HDL cholesterol tends to be transported away from arteries towards the liver, en route to being passed from the body. It has also been theorised that HDL cholesterol slows the growth of plaque by removing excess LDL cholesterol build up from the plaque. Contrary to LDL cholesterol, high levels of HDL cholesterol reduces the risk of heart disease and stroke [10].
Where does cholesterol come from?
Cholesterol comes from outside and inside the human body:
- Inside – The liver produces approximately 1000mg of cholesterol per day (it also helps to remove any excess cholesterol in the blood). This is approximately 80% of the cholesterol present in the body [7].
|
|---|
| [11] |
- Outside – Food from plants doesn’t contain cholesterol, however, food obtained from animals does [10].
In general, the body does not need any cholesterol from outside because it makes all the cholesterol that it needs within the body [10]. If too much cholesterol is present in the bloodstream, hypercholesterolemia can result, increasing the risk of heart disease . Saturated fatty acids and trans fats are the main causes of rising cholesterol levels in the blood [10]. Cholesterol levels also tend to rise with age [12].
How does the body make cholesterol?
Cholesterol synthesis is a long, complex process. It involves several stages of the formation and subsequent conversion of intermediate compounds. This is a brief summary:
- Acetyl-CoA (CoA = coenzyme) and acetoacetyl-CoA undergo condensation to form Hydroxymethylglutaryl-coenzyme A (HMG-CoA). The process is catalysed by HMG-CoA Synthase.
- HMG-CoA Reductase then catalyses the reduction of HMG-CoA to mevalonate. This is achieved by using NADPH to reduce the carboxyl group of hydroxymethylglutarate (HMG) (connected to the thiol of CoA via an ester linkage) to an aldehyde, followed by reduction to an alcohol. This reaction is the rate determining step of the whole cholesterol synthesis reaction and as such, is the reaction targeted by pharmaceutical drugs designed to control cholesterol levels in the body.
- The next stage involves two phosphate transfers from 2 ATP molecules to allow phosphorylation of the newly formed mevalonate molecule, giving a new pyrophosphate derivative. This derivative then undergoes ATP-dependent decarboxylation, with dehydration, catalysed by Pyrophosphomevolanate Decarboxylase, to form a compound called isopentenyl pyrophosphate. This newly formed compound is characterised as an isoprenoid because it is a derivative of isoprene (pictured).
- Isopentenyl pyrophosphate is then interconverted into dimethylallyl pyrophosphate by isopentenyl Pyrophosphate Isomerase, involving protonation and subsequent de-protonation. This means that there will be some molecules of isopentenyl pyrophosphate and some of dimethylallyl pyrophosphate present.
- Next, isopentenyl pyrophosphate undergoes a condensation reaction, catalysed by Prenyl Transferase, with the newly formed dimethylallyl pyrophosphate to yield a single geranyl pyrophosphate molecule. The geranyl pyrophosphate molecule then proceeds to undergo another condensation reaction with an isopentenyl pyrophosphate molecule, resulting in farnesyl pyrophosphate.
- The next stage involves synthesis of a molecule of squalene, made by condensing 2 molecules of farnesyl pyrophosphate together. This condensation reaction involves reduction of NADPH and is catalysed by the catalyst Squalene Synthase.
- Squalene is oxidised to 2,3-oxidosqualene (an epoxide), with the help of Squalene epoxidase (catalyst), NADPH (reductant) and O2 (oxidant, 2 molecules needed).
- Protonation of 2,3-oxidosqualene triggers a sequence of electron shifts, catalysed by Squalene Oxidocyclase, resulting in cyclization. This leads to the generation of the sterol lanosterol.
- The final step in cholesterol synthesis involves 19 sequential reactions, catalysed by enzymes found in the endoplasmic reticulum membranes, which convert lanosterol to the final cholesterol molecule. Diagrams and details of these reactions [13]
The full synthesis, as well as diagrams that make the above description clearer and easier to understand, can be found here [14]
Lowering your blood cholesterol levels
Eating more fruits and vegetables can help, as these foods do not contain any cholesterol [12].
Any form of physical activity can help to reduce cholesterol levels by increasing HDL cholesterol and hence lower the risk of heart disease. HDL cholesterol levels can also be increased through moderate drinking of alcohol – although drinking too much can result in dangers which outweigh the benefits [10].
Unsurprisingly, smoking has the opposite and undesirable effect of raising blood cholesterol levels by lowering HDL cholesterol levels and the secondary effect of causing your blood to clot more readily [10].
Spectra

|
|---|
| [15] |

|
|---|
| [16] |

|
|---|
| [17] |
References
- ↑ Journal; Barton,D.H.R. et al.; JCPRB4; J. Chem. Soc. Perkin Trans. 1; EN; 1973; 599-603.
- ↑ Journal; Freire; Ribas; ANQUBU; An. Quim.; 71; 1975; 418,421.
- ↑ Journal; Vochten; Joos; JCPBAN; J. Chim. Phys. Phys. Chim. Biol.; 67; 1970; 1372,1375.
- ↑ Journal; Smith; Deavenport; JOMRA4; J. Magn. Reson.; 6; 1972; 257.
- ↑ Journal; Kasuga, Kazuyuki; Hatakeyama, Hyoe; Hatakeyama, Tatsuko; MCLCA5; Mol. Cryst. Liq. Cryst.; EN; 147; 1987; 1-14.
- ↑ Journal; Adams, Nathan W.; Bradshaw, Jerald S.; Bayona, Jose-Maria; Markides, Karin E.; Lee, Milton L.; MCLCA5; Mol. Cryst. Liq. Cryst.; EN; 147; 1987; 43-60.
- ↑ 7.0 7.1 http://heart.health.ivillage.com/cholesterol/cholesterol.cfm
- ↑ 8.0 8.1 8.2 8.3 http://chemfinder.cambridgesoft.com/result.asp
- ↑ 9.0 9.1 9.2 9.3 www.scienceclarified.com/Ca-Ch/Cholesterol.html
- ↑ 10.00 10.01 10.02 10.03 10.04 10.05 10.06 10.07 10.08 10.09 http://www.americanheart.org/presenter.jhtml?identifier=4488
- ↑ http://pharmalicensing.com/.../1128004281_433bfab90eaa8.JPG
- ↑ 12.0 12.1 http://www.nlm.nih.gov/medlineplus/cholesterol.html
- ↑ Journal; Risley, John M. Cholesterol biosynthesis: lanosterol to cholesterol. Journal of Chemical Education (2002), 79(3), 377-384. CODEN: JCEDA8 ISSN:0021-9584. CAN 136:290557 AN 2002:149495 CAPLUS
- ↑ http://www.rpi.edu/dept/bcbp/molbiochem/MBWeb/mb2/part1/cholesterol.htm
- ↑ http://webbook.nist.gov/cgi/cbook.cgi?ID=C57885&Units=SI&Type=IR-SPEC&Index=1#IR-SPEC
- ↑ http://webbook.nist.gov/cgi/cbook.cgi?ID=C57885&Units=SI&Mask=200#Mass-Spec
- ↑ http://webbook.nist.gov/cgi/cbook.cgi?ID=C57885&Units=SI&Mask=400#UV-Vis-Spec
