It07:Capsaicin
Capsaicin
<>>| It07:Capsaicin | |
|---|---|
| |
| General | |
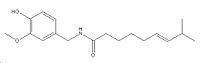
| Systematic name | 8-Methyl-N-vanillyl-trans-6-nonenamide |
| Other names | Capsaicin |
| Molecular formula | C18H27NO3 |
| SMILES | COc1cc(CNC(=O)CCCC/C=C/C(C)C)ccc1O |
| Molar mass | 305.42 |
| Appearance | {{{Appearance}}} |
| CAS number | 404-86-4 |
| Properties | |
| Density & phase | {{{Density}}} g/cm³ |
| Solubility in liqiud carbon dioxide | 0.543648gl-1 (35°C)
pressure dependent |
| Melting point | 339.25K in petroleum ether solvent |
| Boiling point | 483.15-483.15K |
| Acidity (pKa) | {{{pKa}}} |
| Basicity (pKb) | {{{pKb}}} |
| Chiral rotation [α]D | {{{Rotation}}}° |
| Viscosity | {{{Viscosity}}} cP at 25°C |
| Structure | |
| Molecular shape | {{{Mol_Shape}}} |
| Coordination geometry |
{{{Coordination}}} |
| Crystal structure | Monoclinic |
| Dipole moment | {{{DM}}} D |
| Hazards | |
| MSDS | [External MSDS][1] |
| Main hazards | Irritant |
| NFPA 704 | {{{NFPA}}} |
| Flash point | {{{Fp}}}°C |
| R/S statement | R: {{{R-S}}} S: ? |
| RTECS number | {{{RTECS}}} |
| Supplementary data page | |
| Structure and properties |
n, εr, etc. |
| Thermodynamic data |
Phase behaviour Solid, liquid, gas |
| Spectral data | UV, IR, NMR, MS |
| Related compounds | |
| Other anions | {{{Other_anion}}} |
| Other cations | {{{Ohter_cation}}} |
| Related compounds | {{{Relative_Compounds}}} |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox disclaimer and references | |
| Structure | |
|---|---|
| Molecular shape | {{{MolShape}}} |
| Coordination geometry |
{{{Coordination}}} |
| Crystal structure | {{{CrystalStruct}}} |
| Dipole moment | {{{Dipole}}} D |
Capsaicin is a colourless crystalline alkaloid and also one of the most pungent compounds. Capsaicin is irritant to skin and mucous membrane. This compound is slightly soluble in water but is very soluble in organic solvents such as alcohol and fats. It is a part of capsaicinoid which is an active component of capsicum (chilli pepper’s biological name). Capsaicin of capsaicinoid is the main source for the chilli peppers being hot. Natural occurring capsaicinoid consists of six different compounds, capsaicin, dihydrocapsaicin which are the main components. The minor components of capsaicinoid are nordihydrocapsaicin, homocapsaicin and homodihydrocapsaicin.[2] [3]
(E)-N-[(4-hydroxy-3-methoxyphenyl)methyl]-8-methyl-6-nonenamide |
Capsaicinoid
As it is stated above, the capsaicinoid in chilli pepper consists of six natural occurring compounds. All of which are pungent but each compound has different level of pungency and irritations. Nordihydrocapsaicin is class as the least irritating and least pungent out of all six. The most irritating and pungent molecule is homodihydrocapsaicin. This compound is said to cause burning sensations which is so strong that you even feel your tongue. Rest of them are rated midst, however, since capsaicin and dihydrocapsaicin have the highest composition of capasnoid, they are rated very irritating on overall effect.
Toxicity and lethal dose
Pure capsaicin needs to be handled with extreme care in the labs. Due to irritating nature of capsaicin, it needs to be handled in air filtered room and the person handling it needs to wear full body protection in order to prevent the inhalation of capsain. When a person inhales it, the person would suffer from severe burning and irritation. At high concentration of capsaicin, it disturbs the cells from producing neurotransmitters which causes the feeling of numbness and eventually the cells will be damaged or destroyed at very high concentration. In the guinea pig experiment, it is found out that the lethal dose varies depending on the way capsaicin is taken in to body. When it is given by injection, the lethal dose is 56 milligram. When it is given by consumption, 190 milligram is the lethal dose. When it is absorbed through skin, the lethal dose is 512 milligrams. It is found that different animals have different capacity of capsaicin dose.
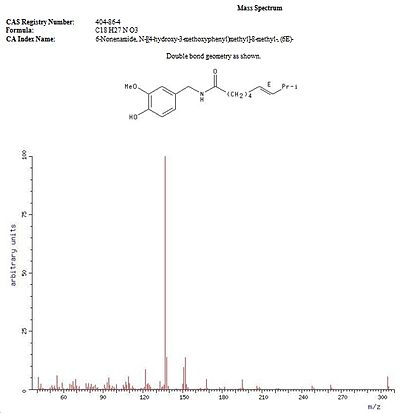
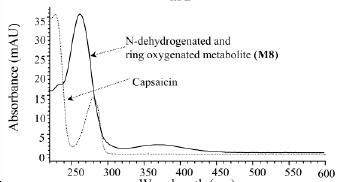
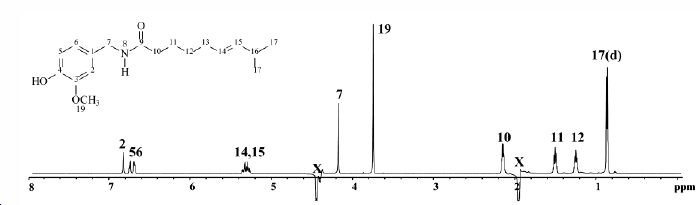
Spectrum Data
Mass Spectrum
UV Spectrum
1H NMR spectrum
Reference
- ↑ http://www.sciencelab.com/xMSDS-Capsaicin_Natural-9923296
- ↑ http://www.fiery-foods.com/dave/capsaicin.asp
- ↑ Journal of Chemical Ecology, Vol. 32, No. 3, March 2006 DOI:10.1007/s10886-005-9017-4
- ↑ WSS: Spectral data were obtained from Wiley Subscription Services, Inc. (US)
- ↑ Chem. Res. Toxicol., 16 (3), 336 -349, 2003 DOI:S0893-228x(02)05599-6 10.1021/tx025599q S0893-228x(02)05599-6
- ↑ Chem. Res. Toxicol., 16 (3), 336 -349, 2003 DOI:S0893-228x(02)05599-6 10.1021/tx025599q S0893-228x(02)05599-6