It07:Capreomycin
Capreomycin
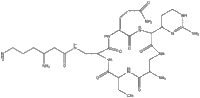
Capreomycin is used in the treatment of Tuberculosis (TB) but its use is limited due to harmful side effects. Capreomycin is found in the aminoglycoside family of antibiotics. It has a molecular formula of C25H44N14O8.
Structure
| It07:Capreomycin | |
|---|---|
| |
| General | |
| Other names | Capreomycin |
| Molecular formula | C25H44N14O8 |
| SMILES | NC(CC(NCC2NC(C(CO)NC
(C(N)CNC(C(NC(/C(NC2=O) =C\CC(N)=O)=O)C1N=C(N) NCC1)=O)=O)=O)=O)CCCN |
| Molar mass | 668.71 |
| Properties | |
| Storage Temperature | -200 |
| Hazards | |
| MSDS | http://www.sigmaaldrich.com/catalog/search/ProductDetail/SIGMA/C4142 Sigma Aldrich Safety Data |
| Main hazards | May cause damage to an the unborn child,
May be harmful |
| Supplementary data page | |
| Structure and properties |
n, εr, etc. |
| Thermodynamic data |
Phase behaviour Solid, liquid, gas |
| Spectral data | UV, IR, NMR, MS |
| Related compounds | |
| Other anions | {{{Other_anion}}} |
| Other cations | {{{Ohter_cation}}} |
| Related compounds | {{{Relative_Compounds}}} |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox disclaimer and references | |
| Structure | |
|---|---|
| Molecular shape | {{{MolShape}}} |
| Coordination geometry |
{{{Coordination}}} |
| Crystal structure | {{{CrystalStruct}}} |
| Dipole moment | {{{Dipole}}} D |
Overview
Capreomycin has the ability to kill a wide variety of bacteria. It does this by binding to the bacterial cell which results in the formation of abnormal proteins. Proteins are necessary for the bacteria's survival and thus the abnormal proteins will kill the bacteria. The bacteria responsible for TB is difficult to kill and so capreomycin is prescribed in combination with other medications that target the bacteria in different ways. It is risky to treat TB using capreomycin alone as the bacteria can develop resistance. Although other medications are used with capreomycin these must be carefully chosen: if used in conjunction with other injectable TB treatments (streptomycin and viomycin) there is an increased risk of hearing and kidney damage in the patient. Capreomycin is only used when the bacteria are resistant to the drugs that are usually prescribed to treat TB.
Adverse Effects
The following are some of the side effects that are known to be associated with this medicine.
• Skin irritation and itching (pruritus)
• Allergy to active ingredients (hypersensitivity)
• Rash (allergic reaction)
• Loss of hearing
• Damage to the kidneys
• Alteration in results of liver function tests
• Disruption to the chemical components balance of electrolytes in the blood
Further Studies
Capreomycin is an important drug used for TB which has developed resistance to many drugs. This is a characteristic of TB drugs and is termed multi-drug resistance (MDR). Capreomycin displays a unique bactericidal effect on non-replicating TB bacilli among known anti-TB drugs.
In a 2005 journal by L. Heifets, J. Simon and V. Pham it showed that only capreomycin is active against non-replicating M. tuberculosis bacilli at the same level as metronidazole in an in vitro model of persistence that monitors the curve of decline in bacterial counts. This confirms the conclusion made in a 1996 journal by N. Rastogi, V. Labrousse and K.S. Goh that stated that capreomycin was effective against MDR and intracellular TB bacilli.
Synthesis
The synthesis of tuberculostatic macrocyclic peptide antibiotic capreomycin IB is composed of 27 complex steps and was determined in 2003 and outlined in a journal written by Duane E. DeMong and Robert M. Williams of Colorado State UniVersity. An enolate-aldimine condensation takes place between a chiral glycine aluminum enolate and the benzyl imine of 3-tert-butyldimethylsiloxy-propanal. This produces the cyclic guanidine amino acid (2S,3R)-capreomycidine. Finally there is a Hofmann rearrangement on a late-stage pentapeptide in order to transform an asparagine residue into a diaminopropanoic acid residue.
Advancements
The August 2007 issue of 'Antimicrobial Agents and Chemotherapy' http://aac.asm.org/ reported a new novel way of treating TB. The findings, also reported by Reuters http://www.medscape.com/viewarticle/562095, presents treatment by inhalation of large porous particles of capreomycin following studies using a guinea pig model. Dr. Anthony J. Hickey and his research team at the University of North Carolina introduced this new concept as a way of the drug being adminstered so that it can directly act at the site of origin thereby hopefully reducing the severe side effects.
In the report the guinea pigs were tested using different methods. The effects of these methods were measured by looking at lung histopathologies for a reduction of the bacteria present in the lungs and by wet tissue weights. It was found that the lungs of animals receiving capreomycin particles by passive inhalation showed significantly, by histopathology, a smaller degree of inflammation and bacterial burdens than the animals treated by other methods.
As a result of this study, it was concluded that treating TB by means of inhaled capreomycin is feasable and safe.
References
1. L. Heifets, J. Simon and V. Pham, Capreomycin is active against non-replicating M. tuberculosis, Ann Clin Microbiol Antimicrob 4 (2005) (1), p. 6. http://www.ann-clinmicrob.com/content/4/1/6
2. N. Rastogi, V. Labrousse and K.S. Goh, In vitro activities of fourteen antimicrobial agents against drug susceptible and resistant clinical isolates of Mycobacterium tuberculosis and comparative intracellular activities against the virulent H37Rv strain in human macrophages, Curr Microbiol 33 (1996) (3), pp. 167–175 http://www.springerlink.com/content/rhkdjf3lngt1tnnd/
3. Synthesis D. E. DeMong, Journal of the American Chemical Society, 2003, 125, 8561-8565. http://pubs.acs.org/cgi-bin/article.cgi/jacsat/2003/125/i28/pdf/ja0351241.pdf
4. Inhaled Large Porous Particles of Capreomycin for Treatment of Tuberculosis in a Guinea Pig Model Garcia-Contreras L, Fiegel J, Telko MJ, Elbert K, Hawi A, Thomas M, VerBerkmoes J, Germishuizen WA, Fourie PB, Hickey AJ, Edwards D. University of North Carolina, Chapel Hill, NC 27599-7360, USA. http://aac.asm.org/cgi/content/full/51/8/2830?view=long&pmid=17517845
