It07:Capecitabine
Capecitabine
| It07:Capecitabine | |
|---|---|
| |
| General | |
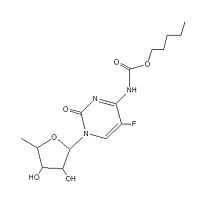
| Systematic name | pentyl N-[1-[(2R,3R,4S,5R)-3,4-dihydroxy-5-methyloxolan-2-yl]-5-fluoro-2-oxopyrimidin-4-yl]carbamate |
| Other names | Xeloda |
| Molecular formula | C15H22FN3O6 |
| SMILES | CCCCCOC(=O)NC1=NC(=O)N(C=C1F)C2C(C(C(O2)C)O)O |
| Molar mass | 359.350083 g/mol |
| CAS number | 154361-50-9 |
| Structure | |
|---|---|
| Molecular shape | {{{MolShape}}} |
| Coordination geometry |
{{{Coordination}}} |
| Crystal structure | {{{CrystalStruct}}} |
| Dipole moment | {{{Dipole}}} D |
3D Structure
Capecitabine
Capecitabine, trade name Xeloda, is an orally administered prodrug (drug which is administered in an inactive form and is metabolised in vivo to an active metabolite) of Fluoroacil (FU), one of the most frequently used anti tumour agents for treating solid tumours. Capecitabine is used in the treatment of colorectal and metastatic breast cancer. It can be used in monotherapy or more commonly in combination therapy usually with doxcetaxel.
Capecitabine is ingested and only gets converted to its active metabolite (FU) by thymidine phosphorylase.[1] This is useful as higher levels of this enzyme are found in tumours and the liver as opposed to normal healthy tissue.
The most common side effects occurring in patients receiving monotherapy are:
- Lymphopenia
- Anaemia
- Diarrhoea
- Hand and Foot syndrome
- Nausea
- Fatigue
- Hyperbilirubinaemia
- Dermatitis
- Vomiting
Synthesis
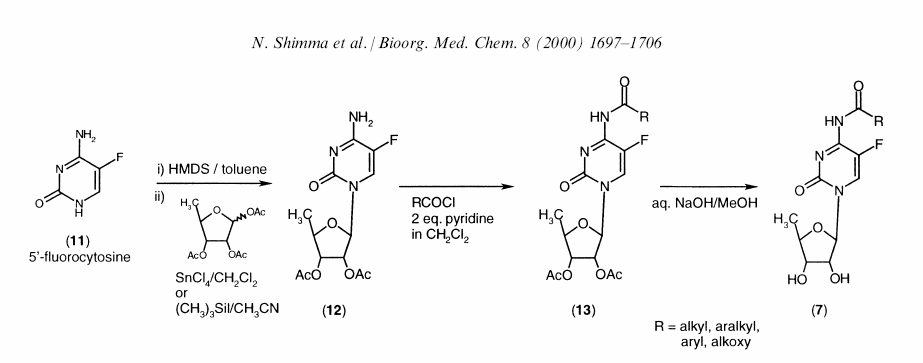
The synthesis takes place in three steps [2]
- Glycosidation of 11 with 1,2,3-tri-O-acetyl-5-deoxyribose with stannic tetrachloride in dichloromethane.
- Acylation of the N4 amino group with acid chlorides in pyridine/dichloromethane.
- Alkaline Hydrolysis of the acetyl group
In the reaction scheme above R = O(CH2)4CH3
Dose
Combination Therapy with Capecitabine 1,250 mg/m(2) twice daily for two weeks then a week of no treatment. This is a cycle of treatment. On the first day of each cycle a intravenous docextaxel dose of 75mg/m(2) is given. [3]