It07:Bufotoxin
Bufotoxin
| Bufotenin | |
|---|---|
| |
| General | |
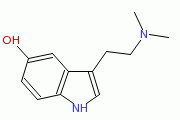
| Systematic name | 3-(2-Dimethylamino-ethyl)-1H-indol-5-ol |
| Molecular formula | C12H16N2O |
| SMILES | OC1=CC2=C(NC=C2CCN(C)C)C=C1 |
| Molar mass | 204.27 gmol-1 |
| Properties | |
| Melting point | 419.65 K [1] |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox disclaimer and references | |
Bufotoxin is a composition of toxins that are used by some toads (genus Bufo). It can be found on the skin of the toads as well as in the venom and the parotoid glands. The toxin consists of a mixture of:
5-MeO-DMT, bufagins, bufotalin, bufotenine, bufothionine, epinephrine, norepinephrine, and serotonin.
The composition of the toxin depends where exactly the toxin comes from. Some plants mushrooms and amphibians also use the toxin.
Bufotenin (also Bufotenine)
Bufotenin (a typtamine alkaloid)is used by some animals for there hallucinogenic ability in a defence system. It is found in mushrooms, some plants, mammals and possibly in bodily fluids of schizophrenics.
3D representation of Bufotenin molecule
3D model of Bufotenin unit cell
Pentahelicene |
Spectral data
Infrared spectrum
IR peaks (Nujol): 3620 cm-1 journal [2]
NMR
1H NMR peaks (D2O): adapted from journal[3]
| Chemical shift | Splitting multiplicity | Integration | Coupling constant (J/Hz) |
|---|---|---|---|
| 2.76 | singlet | 6H | N\A |
| 2.95 | triplet | 2H | 7.5 |
| 3.18 | triplet | 2H | 7.5 |
| 6.86 | double doublet | 1H | 8.8, 2.6 |
| 7.02 | doublet | 1H | 2.6 |
| 7.15 | singlet | 1H | N\A |
| 7.38 | doublet | 1H | 8.8 |
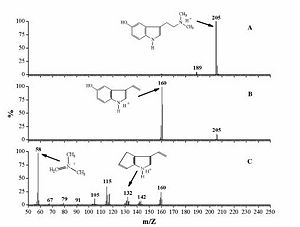
Mass Spectrum
Human experience of Bufotenin vapour
After inhaling the fumes from the bufotenin the inital effect is an increased intensity of visuals and and anxiety accompanied by a general increased awareness. The next step in the experience is hallucinating and seeing swirling around the room with sweating. After a while the sweating and anxiety stops and a more relexed atmosphere still with strong hallucinating effects occurs. After a couple of hours the effect wears off into nothing. The effect of the bufotenin can depend on which form the bufotenin is in e.g. calcium bufotenin or bufotenin hydrochloride can have very different effects.[5]
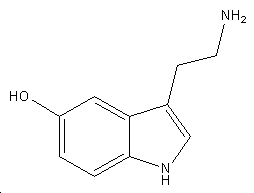
Serotonin
| Serotonin | |
|---|---|
| |
| General | |
| Systematic name | 3-(2-aminoethyl)-1H-indol-5-ol |
| Molecular formula | C10H12N2O |
| [1] | 5202 |
| [2] | 50-67-9 |
| SMILES | OC1=CC=C(NC=C2CCN)C2=C1 |
| Molar mass | 176.215 gmol-1 |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox disclaimer and references | |
3D Representation of Serotonin
Serotonin |
Serotonin picrate monohydrate Crystal Unit Cell (C10H13N2O+, C6H2N3O7-, H2O)
Serotonin |
References
- ↑ I. J. Pachter, D. E. Zacharias and O. Ribeiro, J. Org. Chem., 1959, 24, 1285-1287.DOI:10.1021/jo01091a032
- ↑ G. Revial, I. Jabin, S. Lim and M. Pfau, J. Org. Chem., 2002, 67, 2252-2256.DOI:10.1021/jo0110597
- ↑ G. Revial, I. Jabin, S. Lim and M. Pfau, J. Org. Chem., 2002, 67, 2252-2256.DOI:10.1021/jo0110597
- ↑ T. O. G. Costa, R. A. V. Morales, J. P. Brito, M. Gordo, A. C. Pinto and J. C. Bloch, Toxicon, 2005, 46, 371-375. DOI:10.1016/j.toxicon.2005.02.006
- ↑ Erowid experience vaults:http://www.erowid.org/experiences/exp.php?ID=64707
