It07:Azulene
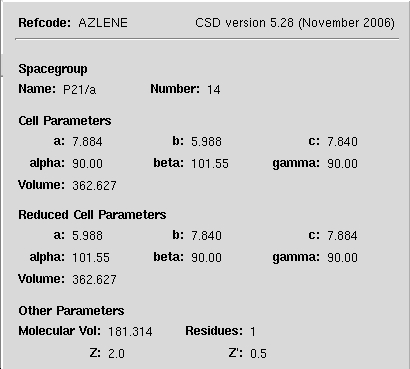
3D structure and crystal unit cell
1
3D structure of azulene.
Right click on molecule to explore
| It07:Azulene | |
|---|---|

| |
| General | |
| Systematic name | Azulene |
| Other names | Azulene |
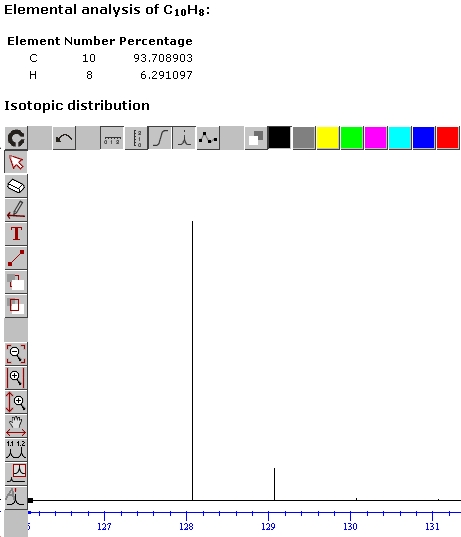
| Molecular formula | C10H8 |
| SMILES | C12=CC=CC=CC1=CC=C2 |
| Molar mass | 128.06 |
| Appearance | blue/purple crystals |
| CAS number | {{{275-51-4}}} |
| Properties | |
| Density & phase | 1.18 g/cm³ |
| Solubility in water | {{{Sol_Water}}} g/100 ml (25°C) |
| Melting point | 99oC |
| Boiling point | 242oC |
| Acidity (pKa) | {{{pKa}}} |
| Basicity (pKb) | {{{pKb}}} |
| Chiral rotation [α]D | {{{Rotation}}}° |
| Viscosity | {{{Viscosity}}} cP at 25°C |
| Structure | |
| Molecular shape | {{{Mol_Shape}}} |
| Coordination geometry |
{{{Coordination}}} |
| Crystal structure | {{{Crystal_Structure}}} |
| Dipole moment | {{{DM}}} D |
| Refractive index | 1.4969 wavelength=689.3 nm 25oC |
Crystal unit cell structure.
Right click on molecule to explore
Unit cell parameters in Å
About azulene
point group = C2v 2
Azulene is an isocyclic compound and it belongs to the class of compounds called monterpenes. Monterpenes have two isoprene units and have the molecular formula C10H16. It’s also an isomer of naphthalene. In 1937 Placidus Plattner was the first person to successfully synthesise azulene. It is a pretty looking compound in its solid state. It’s a blue/purple crystalline solid at room temperature and pressure.
Azulene is used in cosmetics and in many pigments due to its striking colour. Interestingly enough, this compound can be obtained by steam distillation of chamomile! (and other natural sources such as yarrow which is a plant that grows in the northern hemisphere) This method of obtaining azulene was discovered in the 15th century. It is a 10 pi electron system and has aromatic properties similar to benzene due to the presence of conjugated C=C bonds. It is interesting to note that since the whole molecule is not aromatic (due to the presence of a C-C bond in the middle adjoining the 2 cyclic structures. This C-C single bond is present in all kekule structures of azulene), the stabilisation gain is only about half as much as naphthalene.
Synthesis
3
Isotopic distribution
4
Spectra
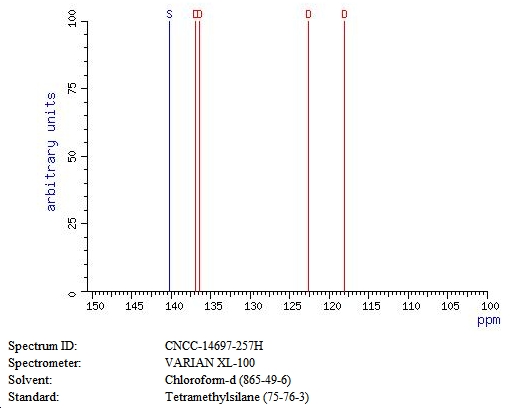
13C spectrum 6
Mass specrum 7
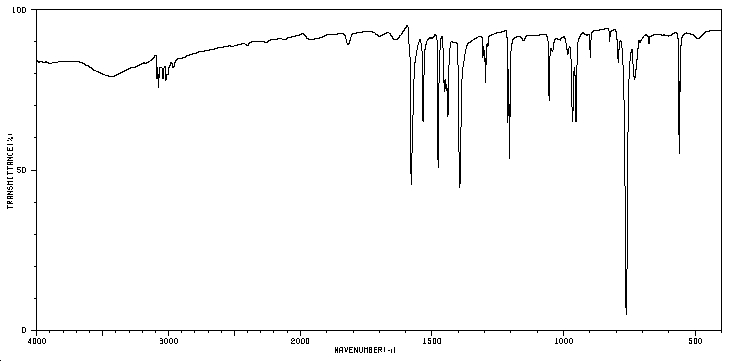
Infra red spectrum 8
Stability
The stabilising delocalisation component of the molecule means that it is relatively stable. However, it is combustible like with a lot of alkenes as well as being incompatible with strong oxidizing agents. Azulene can be safely transported by means of air, sea and road freight.
Hazards and safety
5
- Irritating to eyes.
- Irritating to respiratory system.
- Irritating to skin.
- In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
- Wear suitable protective clothing
References
- 1)DOI reference
- 2)DOI reference
- 3)DOI reference
- 4)http://www.chemcalc.org/
- 5)http://physchem.ox.ac.uk/MSDS/AZ/azulene.html
- 6)Carbon-13 chemical shifts and substituent effects in methyl azulenes. Braun, S.; Kinkeldei, J. Inst. Org. Chem., Tech. Hochsch. Darmstadt, Darmstadt, Fed. Rep. Ger. Tetrahedron (1977), 33(14), 1827-32. CODEN: TETRAB ISSN: 0040-4020. Journal written in German. CAN 88:49814 AN 1978:49814 CAPLUS
- 7)Energy transfer of highly vibrationally excited azulene. III. Collisions between azulene and argon. Liu, Chen-Lin; Hsu, Hsu Chen; Lyu, Jia-Jia; Ni, Chi-Kung. Institute of Atomic and Molecular Sciences, Academia Sinica, Taipei, Taiwan. Journal of Chemical Physics (2006), 125(20), 204309/1-204309/6. Publisher: American Institute of Physics, CODEN: JCPSA6 ISSN: 0021-9606. Journal written in English. CAN 146:149461 AN 146:149461 CAPLUS
- 8)Integrated spectral database system of organic compounds