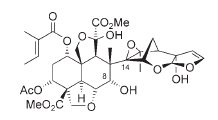
It07:Azadirachtin
Azadirachtin
| It07:Azadirachtin | |
|---|---|

| |
| Systematic name | {{dimethyl (2aR,3S,4S,4aR,5S,7aS,8S,10R,10aS,10bR)-10-acetoxy-
3,5-dihydroxy-4-[(1aR,2S,3aS,6aS,7S,7aS)- 6a-hydroxy-7a-methyl-3a,6a,7,7a-tetrahydro- 2,7-methanofuro[2,3-b]oxireno[e]oxepin- 1a(2H)-yl]-4-methyl-8- {[(2E)-2-methylbut-2-enoyl]oxy}octahydro- 1H-naphtho[1,8a-c:4,5-b'c']difuran- 5,10a(8H)-dicarboxylate}} |
| Chemical formula | C35H44O16 |
| Molar mass | 720.63 g/mol |
| Density | 1.51 ± 0.1 g/cm³ |
| Solubility | 0.015 g/dm3 |
| Boiling point | 792.4 ± 60.0 °C |
| CAS number | 11141-17-6 |
| SMILES | C\C=C(/C)\C(=O)O[C@H]1C[C@H]
([C@]2(CO[C@@H]3[C@@H]2[C@]14CO[C@@] ([C@H]4[C@]([C@@H]3O)(C)[C@@]56[C@@H] 7C[C@H]([C@@]5(O6)C)[C@]8(C=CO[C@H]8O7)O) (C(=O)OC)O)C(=O)OC)OC(=O)C |
| Hazards | Possible irritant, toxicological properties have not been investigated. |
| Disclaimer and references | |
Azadirachtin is a triterpenoid compound extracted from the Neem tree (Azadirachta indica). The molecular structure is highly complex, with 16 stereogenic centers - seven of which are tetrasubstituted. Complete synthesis was first achieved by Steven Ley in 2007, 22 years after the structure was established (also by Ley and co-workers) in 1985.[1] It is a yellow-green powder, with a strong garlic-sulfur odor.[2][3]
Mode of Action
The process of moulting (shedding of exoskeleton) and metamorphosis (final transformation where insect makes transition from pupal stage to become fully adult) is triggered by prothoracicotropic hormone (PTTH) as well as ecdysone.[4] Azadirachtin blocks the action of ecdysone and hence stops moulting and subsequent growth of insects. As such, it is a good antifeedant - a substance that inhibits the insect from feeding but does not kill it directly.[5]
3d Structure
To zoom in/out, click on molecule and use scroll wheel on mouse.
Sources and Synthesis
The Azadirachtin molecule has a complex molecular structure which is mainly the reason why it has taken so long for a successful synthesis path to be found. There are 16 continuous stereogenic centres, seven of which are tetrasubstituted carbons. It is sensitive to both acid and base and is unstable in light. The C8-C14 bond is of particular importance as it was the hardest bond to create given the highly hindered environment around it. There are also intra-molecular hydrogen-bonds which impose even more conformational considerations when considering a synthetic path.
The synthetic path can be summarised as the fusing of a pyran and decalin fragment by selective O-coupling. The crucial C8-C14 bond is then formed under thermal or gold(I)-catalysed conditions via a Claisen rearrangement. The tricyclic system is then formed by a radical cyclization. The final step involves a selective epoxidation. This was also a challenge to the acheive synthetically, but on prolonged heating with magnesium monoperoxyphthalate in the presence of a radical inhibitor, complete synthesis of Azadirachtin was achieved.
Azadirachtin can also be easily extracted from the seeds of the Neem tree. A polar solvent such as water, ethanol or methanol will suffice. In fact, Indian villagers have been known to crush the seeds and soak them in cold water. After soaking overnight, the emulsion at the top is applied directly to plants.
The exact biosynthesis of Azadirachtin is unknown but most certainly proceeds via a steroid modification. [6]
Uses
Azadirachtin works as an insect growth regulator. It prevents the larval stage molting into an adult. It works by disturbing or inhibiting the development of the eggs, larvae, or pupae, disturbing mating and sexual communication, repelling larvae and adults, and deterring females from laying eggs.
There has been much research into this pesticide and it has been shown that the spray solution of neem oil helps to control common pests like white flies, aphids, scales, mealy bugs, spider mites, locusts, thrips, and Japanese beetles. There are no reported adverse effects to humans, wildlife, or the environment.
References
- ↑ S. V. Ley, A. A. Denholm and A. Wood, Nat. Prod. Rep., 1993, 10, 109. http://www.rsc.org/ejarchive/NP/1993/NP9931000109.pdf (accessed 25 October 2007)
- ↑ Farm Chemicals Handbook. 1995. Meister Publishing Co. Willoughby, OH.
- ↑ Thomson, W.T. Agricultural Chemicals. Book I: Insecticides. 1992. Thomson Publications, Fresno, CA.
- ↑ Kimball, J. W. (9 February 2002) 'Insect Hormones', http://users.rcn.com/jkimball.ma.ultranet/BiologyPages/I/InsectHormones.html (accessed 23 October 2007)
- ↑ K. Munakata, Pure Appl. Chem., 1975, 42, 57.
- ↑ Synthesis of Azadirachtin: A Long but Successful Journey Gemma E. Veitch, Edith Beckmann, Brenda J. Burke, Alistair Boyer, Sarah L. Maslen, and Steven V. Ley