It07:Atenolol
[1]Atenolol is a beta blocking compound and is used to block the involuntary part of the nervous system known as the sympathetic nervous system. As this part of the nervous system is responsible for the stimulation of the heart beat, its suppression by blocking the action of the respective nerves causes a reduction in heart rate. In contrast to many beta blockers, atenolol is β1 selective and therefore blocks receptors corresponding to heart activity specifically.
3d structure
| Template:Atenolol | |
|---|---|
 | |
| General | |
| Systematic name | 2-[4-(2-hydroxy-3-isopropylamino-propoxy)-phenyl]-acetamide
|
| Other names | Atenol, Altol, Corotenol, Tenolol, Xaten |
| Molecular formula | C14H22N2O3 |
| SMILES | CC(NCC(O)COC1=CC=C(CC(N)=O)C=C1)C |
| Molar mass | 266.34 |
| Appearance | white powder |
| CAS number | 29122-68-7 |
atenolol |
History
[2]Atenolol was intended to replace another β blocker known as propranolol to treat hypertension and was introduced in 1976. Whilst sharing a common β blocking property with propranol, it had the advantage of not being able to get through the blood brain barrier a flaw intrinsic of propranol that resulted in side effects such as nightmares. More recently in 2006, Atenolol was downgraded from first to fourth line of treatment following evidence of its inferior performance to other drugs, more significantly when treating elderly patients. It is still however, the most widely used β blocker in the UK.
Applications
Many of the applications of atenolol are, due to its effect of reducing heart rate and force of heart contractions, related to coronary afflictions. These include the treatment of high blood pressure, or hypertension, chest pains, or angina pectoris, and the reduction unusually quick heart rates. Its treatment of angina is a consequence of the causing of reduced demand for oxygen from the heart, as a result of the lower demands from the cardiac muscle, in response to the hearts oxygen demand exceeding its supply as caused by angina.
Side effects
[3]There are numerous side effects that result from atenolol; some are more common and less serious and others occur more rarely but are more severe. Some of the more common side effects include depression, tiredness dizziness and shortness of breath. More scarcely occurring side effects are allergic reactions, vision problems and reversible hair loss. Some of the most serious side effects that could be caused are irregular heartbeat, difficulty in breathing or swallowing and chest pain. Prior to taking the drug there is no way to know which, if any, of the side effects will be developed in any one person therefore it is at the individuals’ discretion to report anything unusual to their medical practitioner.
Synthesis
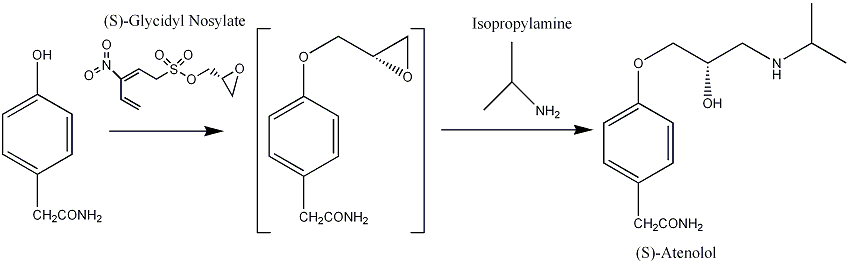
Due to the chiral centre (on the carbon that has the hydroxyl group attached), there are two possible optical isomers of Atenolol. [4] The desirable β-blocking property is associated with the (S) enantiomer and it is speculated that the (R) enantiomer is responsible for the side effects. Consequently syntheses that result in the pure (S) enantiomer have been developed. [5]One such synthesis is as follows :
Spectroscopy and Spectrometry

[6]Infra-red Spectrum of Atenolol
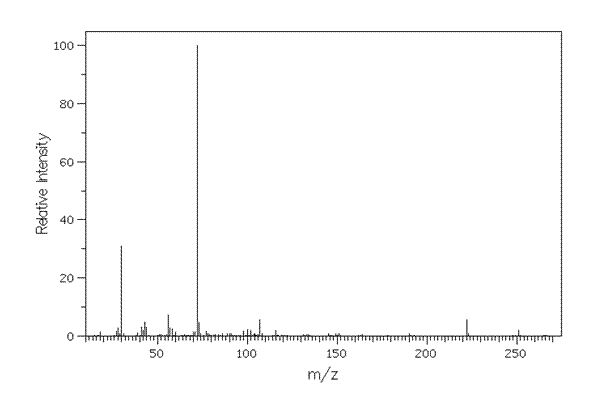
[7]Mass Spectrum of Atenolol
References
- ↑ http://www.medicinenet.com/atenolol/article.htm
- ↑ Atenolol information page [1]
- ↑ Atenolol Side Effects [2]
- ↑ DOI:10.1080/00397919908085905
- ↑ CsF in organic synthesis. Regioselective nucleophilic reactions of phenols with oxiranes leading to enantiopure β-blockers [3]
- ↑ Integrated Spectral Database System of Organic Compounds (Data were obtained from the National Institute of Advanced Industrial Science and Technology (Japan))
- ↑ Integrated Spectral Database System of Organic Compounds (Data were obtained from the National Institute of Advanced Industrial Science and Technology (Japan))
