It07:Astaxanthin
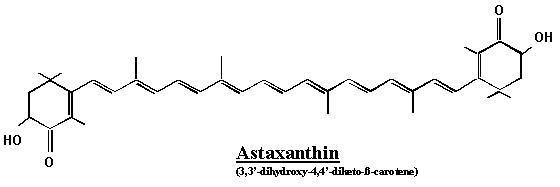
Astaxanthin
| Astaxanthin | |
|---|---|

| |
| General | |
| Systematic name | 6-hydroxy-3-[(1E,3E,5E,7E,9E,11E,
13E,15E,17E)-18-(4-hydroxy-2,6,6- trimethyl-3-oxo-1-cyclohexenyl)- 3,7,12,16-tetramethyloctadeca-1,3, 5,7,9,11,13,15,17-nonaenyl]-2,4, 4-trimethylcyclohex-2-en-1-one |
| Other names | Astaxanthin |
| Molecular formula | C40H52O4 |
| SMILES | C\C(=C\C=C\C=C(\C)/C=C\C=C
(\C)/C=C\C1=C(C)C(=O)C(O)CC1 (C)C)/C=C\C=C(\C)/C=C \C1=C (C)C(=O)C(O)CC1(C)C |
| Molar mass | 596.839 g/mol |
| CAS number | 472-61-7 |
| Properties | |
| Density & phase | {{{Density}}} g/cm³ |
| Solubility in water | {{{Sol_Water}}} g/100 ml (25°C) |
| Melting point | {{{Mp}}} K |
| Boiling point | {{{Bp}}} K |
| Acidity (pKa) | {{{pKa}}} |
| Basicity (pKb) | {{{pKb}}} |
| Chiral rotation [α]D | {{{Rotation}}}° |
| Viscosity | {{{Viscosity}}} cP at 25°C |
| Structure | |
| Molecular shape | {{{Mol_Shape}}} |
| Coordination geometry |
{{{Coordination}}} |
| Crystal structure | {{{Crystal_Structure}}} |
| Dipole moment | {{{DM}}} D |
| Hazards | |
| MSDS | External MSDS |
| Main hazards | {{{Hazards}}} |
| NFPA 704 | {{{NFPA}}} |
| Flash point | {{{Fp}}}°C |
| R/S statement | R: {{{R-S}}} S: ? |
| RTECS number | {{{RTECS}}} |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox disclaimer and references | |
Astaxanthin |
(!) THIS ARTICLE IS NOT A SUBSTITUTE FOR A MEDICAL ADVICE. IF YOU HAVE A SPECIFIC HEALTH CONCERN PLEASE CONSULT A QUALIFIED DOCTOR OR A NUTRITIONIST.
Astaxanthin is a powerful antioxidant which is responsible for the red colour of salmon. Astaxanthin is said to be up to 500 times as strong as vitamin E (a common antioxidant found in vegetable oils). It is sometimes referred to as a “super-oxidant” or vitamin X.
The content of astaxanthin in wild (Pacific) salmon is typically between 14 and 40mg per kg whereas that of farmed Atlantic salmon is between 4 and 10mg per kg. Astaxanthin is also found in trout, crustaceans, and red yeast. Those seafood, however, do not synthesise astaxanthin within their own bodies – rather, they obtain them from planktons that they feed on (astaxanthin is commercially produced by extraction from microalgae).
Chemical Properties
Molecular Weight: 596.839 [1] Melting Point: 210 - 215°C.
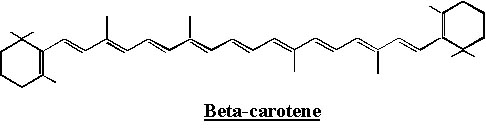
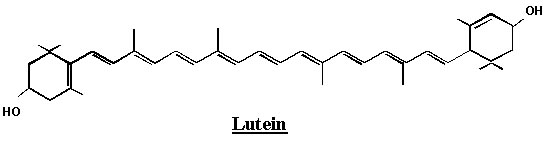
Astaxanthin is not soluble at room temperature but is lipophilic (fat-soluble) and is classified as a cartenoid, along with beta-carotene and lutein. More specifically, it belongs to a class of xantophyll cartenoids.
Astaxanthin has a structure which is similar to that of beta-carotene and lutein, but astaxanthin also contain carbonyl and hydroxyl groups which cause greater polarities and are responsible for the different chemical properties: carbonyl groups are electron withdrawing by resonance and can thus create sites on the carbon chain which are susceptible to nucleophilic attacks.
There are two chiral centres on an astaxanthin molecule, which are labelled 3 and 3’ (the third atoms from the carbonyl oxygen of their respective six membered rings) and so there are three different enantiomers of this compound, namely 3S3’S, 3R3’S, 3R3’R. 3R3’S is not commonly seen in the nature. Due to the nine carbon-carbon double bonds present on the carbon chain between the two carbon rings, geometrical isomerism is shown as well. At each site of double bonds, there could be an E or Z conformation (respectively trans or cis in the old fashioned way of speech). In naturally occurring astaxanthin molecules, Z isomerism has been observed at the carbon atoms 9, 13 and 15, both singly and in combination: 9Z, 13Z, 15Z, 9Z-13Z, 9Z-15Z, 13Z-15Z and 9Z-13Z-15Z. However, all-E is generally the most abundant form in nature and is the most thermodynamically stable since the repulsion between the electron densities is at minimum.
Both the hydroxyl (OH) groups could be esterified in the presence of an acid (for instance a fatty acid): hence we could have an unesterified, mono-esterified and diesterified forms of astaxanthin. It turns out that esterification of the hydroxyl groups further increases the hydrophobicity of the molecule. The unesterified forms of this compound is highly unstable and so the ones found in nature are often esterified or bound to protein molecules.
Extraction
Although astaxanthin is found in many algae, the green algae Haematococcus pluvialis is mainly used as a commercial source since it has the ability to synthesise and store a greater amount of this pigment than others. It has been found that limiting the growth of H. pluvialis aided the development of further astaxanthin.
Oxygen Quenching
Astaxanthin, when it is in crystalline form, is so susceptible to oxidation that it would only require a trace of oxygen in order to decompose. An oxygen molecule is reactive due to its two free radicals (which are believed to be the cause of cancer). Astaxanthin (and other cartenoids) limit the activity of these oxygen molecules by taking away their energy and absorbing it themselves. They then release that energy in the form of heat, relaxing to the ground states.
The quenching constant of astaxanthin is reported to be 2.4 × 1010 Lmol-1sec-1 whereas that of beta-carotene is 1.4 × 1010 Lmol-1sec-1.
Risks
Negative side effects of astaxanthin are not currently known.
References
Choi, Y. E ; Yun, Y.-S.; Park, J. M. Biotechnol. Prog.; (Article); 2002; 18(6); 1170-1175.
Wu, T.-H.; Liao, J.-H.; Hou, W.-C.; Huang, F.-Y.; Maher, T. J.; Hu, C.-C. J. Agric. Food Chem.; (Article); 2006; 54(6); 2418-2423.
Hussein, G.; Sankawa, U.; Goto, H.; Matsumoto, K.; Watanabe, H. J. Nat. Prod.; (Review); 2006; 69(3); 443-449