It07:Aspirin
| It07:Aspirin | |
|---|---|
| |
| General | |
| IUPAC Systematic name | 2-acetoxybenzoic acid |
| Other names | Acetylsalicylic acid |
| Molecular formula | C9H8O4 |
| SMILES | CC(OC1=C(C(O)=O)C=CC=C1)=O |
| CAS number | 50-78-2 |
| Molar mass | 180.16 gmol-1 |
| Properties | |
| Density | 1.40gcm3 |
| Boiling point | 140 °C (284 °F) (decomposes) |
| Melting point | 138-140 °C (280-284 °F) |
| Solubility in water | 10mg / ml (20 °C) |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox disclaimer and references | |
Overview
Aspirin, also known as acetylsalicylic acid, is a salicylate drug which usually uses as an analgesic to relieve pains and minor aches, an antipyretic to reduce fever, or as an anti-inflammatory. Besides this, it also has the antiplatelet effect that causes “blood-thinning” to be used in long term. Aspirin can prevent the heart attack in low doses.
Aspirin was the member of the class of drugs which is known as non-steroidal anti0inflammatory drugs (NSAIDs). Here not all of them are salicylates. But they all have the similar effects.
|
Cyclopentasiloxane |
Synthesis
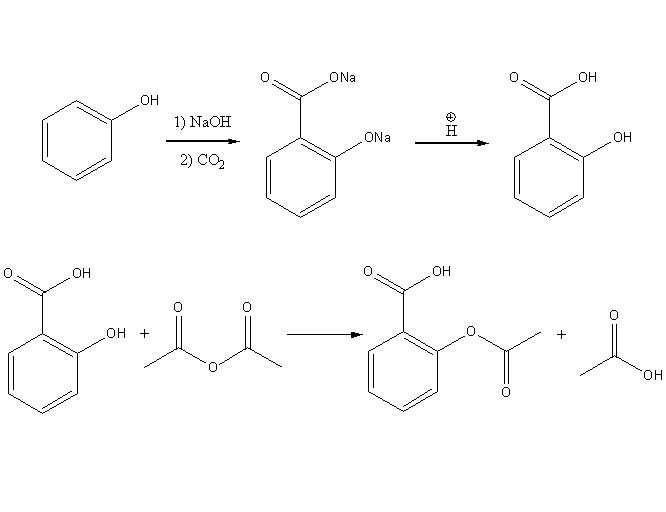
It’s classified as an esterification reaction for the synthesis of aspirin in which the alcohol group from the salicylic acid reacts with the acetic anhydride to form an ester.
Generally, aspirin is synthesized via a two step process. First of all, the phenol that normally generated from coal tar, reacts with a sodium base to form a sodium phenolate. Under high pressure and temperature, it reacts with carbon dioxide to yield salicylate. Finally, it’s acidified to form salicylic acid. This process is called the Kolbe-Schmitt reaction.
Secondly, the salicylic acid is then acetylated via an acetic anhydride, which yields aspirin and acetic acid as a side-product. As it’s hard for the extraction from an aqueous state, this reaction usually produces a low yield. Another method in getting higher yield is to acidify with phosphoric acid, followed by heating under reflux in 40-60 minutes.
In addition, the original synthesis of aspirin from salicylic acid involves acetylation with acetyl chloride. Therefore hydrochloric acid will be formed as a by-product and it is very corrosive and environmentally hazardous. However in the second step above, salicylic acid can be acetylated with acetic anhydride which is a better acylating agent. Then acetic acid formed as the side-product doesn’t contain the harmful properties of the hydrochloric acid. Furthermore, the acetic acid can be recycled. So it’s much better to use acetic anhydride as an acetylating agent.
In the synthesis, a smell of vinegar can be altered if a high concentration of aspirin is used. It is because aspirin could undergo autocatalysis in a moistened condition which then yields salicylic acid and acetic acid.
Therapeutic uses
Aspirin is used commonly in the treatment from mild to moderate pain, including fever and migraines. Next, aspirin is combined with other non-steroidal anti-inflammatory drugs and opioid analgesics in the treatment of pain which is associated with cancer.
Veterinary uses
Aspirin has been used in veterinary medicine to treat pain and arthritis, which can be applied to cats and dogs. But this is not recommended, as there are better medications though. Also, dogs would suffer from gastrointestinal side effects due to salicylate in aspirin.
Moreover, horses can also use aspirin for the pain relief. Again, it is not frequently used because of its relatively short-lived analgesic effects. Also horses do suffer the gastrointestinal side effects once taking aspirin.
Therefore it’s not suggested that aspirin can apply to the animals unless other circumstances.
Side effects
The main side effect of overtaking aspirin is gastrointestinal distress which includes stomach bleeding and ulcers. Due to its anticoagulant property, it causes an increase in bleeding for menstruating women. Moreover, high doses of aspirin will inhibit the synthesis of prothrombin that causes another side effect. And also aspirin is hard to control the symptoms of chickenpox or flu for those children under twelve years old.