It07:Anandamide
Anandamide
| Anandamide | |||
|---|---|---|---|
Anandamide A 3D representation of the 'I' shape conformer. | |||
| Structure | |||
| Identifiers | |||
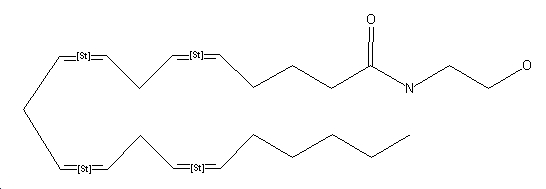
| Systematic name | Eicosa-5,8,11,14-tetraenoic acid (2-hydroxy-ethyl)-amide
(5Z, 8Z, 11Z, 14Z)-N-(2-hydroxyethyl)-5,8,11,14-eicosatetraenamide
| ||
| Other names | Anandamide Arachidonoylethanolamide (AEA) | ||
| Molecular formula | C22H37NO2 | ||
| SMILES | CCCCC\C=C/C\C=C/C\C=C
/C\C=C/CCCC(NCCO)=O | ||
| CAS number | 94421-68-8 | ||
| Properties | |||
| Molar mass | 347.54 gmol-1 | ||
| Appearance | Light yellow oil | ||
| Density & phase | 0.92g/ml | ||
| Solubility in water | {{{Sol_Water}}} g/100 ml (25°C) (soluble in ethanol) | ||
| Melting point | {{{Mp}}} K | ||
| Boiling point | {{{Bp}}} K | ||
| Acidity (pKa) | {{{pKa}}} | ||
| Basicity (pKb) | {{{pKb}}} | ||
| Chiral rotation [α]D | {{{Rotation}}}° | ||
| Viscosity | {{{Viscosity}}} cP at 25°C | ||
| Structure | |||
| Molecular shape | {{{Mol_Shape}}} | ||
| Coordination geometry |
{{{Coordination}}} | ||
| Crystal structure | {{{Crystal_Structure}}} | ||
| Dipole moment | {{{DM}}} D | ||
| Hazards | |||
| MSDS | External MSDS | ||
| Main hazards | {{{Hazards}}} | ||
| NFPA 704 | {{{NFPA}}} | ||
| Flash point | {{{Fp}}}°C | ||
| R/S statement | R: {{{R-S}}} S: ? | ||
| RTECS number | {{{RTECS}}} | ||
| Supplementary data page | |||
| Structure and properties |
n, εr, etc. | ||
| Thermodynamic data |
Phase behaviour Solid, liquid, gas | ||
| Spectral data | UV, IR, NMR, MS | ||
| Related compounds | |||
| Other anions | {{{Other_anion}}} | ||
| Other cations | {{{Ohter_cation}}} | ||
| Related compounds | {{{Relative_Compounds}}} | ||
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox disclaimer and references | |||
Anandamide (a name deriving from the Sanskrit word "ananda," meaning bliss), also known as arachidonoylethanolamide (AEA), is a neurotransmitter present in animal and human organs, commonly in the brain. More specifically, anandamide is a cannabanoid; it binds to the cannabanoid receptor in mammalian brains, the same receptor that the psychoactive ingredient in marijuana targets.
Anandamide as a drug
Anandamide acts primarily as a messenger molecule. It works by binding to CB(1) and CB(2) cannabinoid receptors. The CB(2) receptor is found in the spleen and in some cells in the immune system. CB(1) is found in the intestine, heart and central nervous system. The brain is where the most profound effects can be observed. It has been shown to play a role in pain, appetite regulation, memory, depression and fertility. It is particularly interesting as it allows us to rationalise and explain some aspects of human behaviour on a molecular level. Hence it is a very strong potential drug candidate. Anandamide ‘preservers’ which block Anandamide degradation in the brain (i.e. stops it from being released from the CB(1) receptor site) are currently being researched as drugs. The most promising one is called URB597. It works by inhibiting the enzyme FAAH which breaks down Anandamide in the brain. By blocking the release of Anandamide the concentration of this drug in the neuroreceptors is increased. This enhances the effects of the naturally present Anandamide, without causing the undesirable side effects of THC (present in cannabis) such as memory loss and feeling high. Extensive studies, primarily on rats, carried out by Piomelli and co. at the university of Urbino and Parma show that Anandamide works as a natural anti-depressant. When chronically stressed rats (which have symptoms similar to depression in humans) were treated with URB597 for 5 weeks their behaviour returned to that of normal rats.(Nov. 15 Biological Psychiatry). Thus it has potential as an antidepressant drug. The drug has yet to be tested on humans, but clinical studies will begin this year.[1]
An alternative to this is to stimulate Anandamide building enzymes. The areas of the brain where Anandamide are synthesized naturally by these enzymes are those involved in memory, thought process and movement control. Hence these drugs may well be used to produce different effects to the inhibitors previously discussed.
This area is currently being researched by William A. Devane.
References
|<jmol>
Biosynthesis of Anandamide
There are two ways to synthesise anandamide. One is through the formation from free arachidonic acid and ethanolamine; the other is the formation from N-arachidonoyl phosphatidylethanolamine (PE), through the action of an enzyme called phosphodiesterase. These pathways, however, do not appear to generate a large amount of anandamide under physiological conditions. The generation of anandamide from free arachidonic acid and ethanolamine is catalyzed by a degrading enzyme anandamide amidohydrolase or a fatty acid amide hydrolase working in reverse. This requires large amounts of substrates. For the second pathway, arachidonic acids esterified at the 1-position of glycerophospholipids, which are mostly esterified at the 2-position, are used for the formation of N-arachidonoyl PE, a stored precursor form of anandamide. The reaction schemes of the second pathway are shown in the diagrams below:
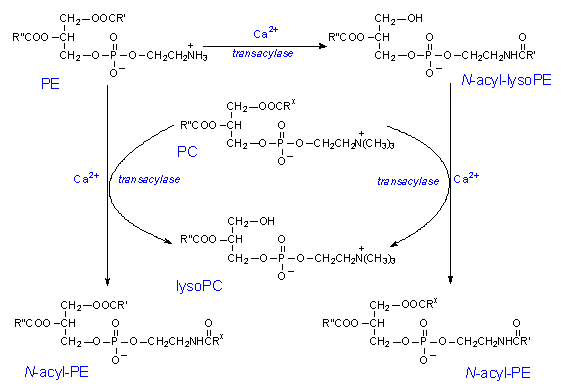
Diagram 1: The synthesis of N-acyl PE through a series of reactions from Ca2+ ions and the enzyme transacylase.
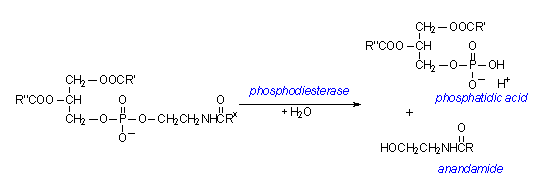
Diagram 2: The second step of the biosynthesis involves the hydrolysis of the N-acyl-phosphatidylethanolamine by the enzyme phosphodiesterase.
Possible Conformers
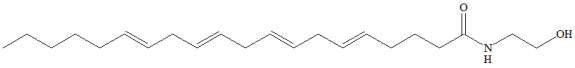
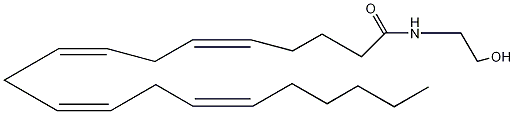
References
1. T. Sugiura, Y. Kobayashi, S. Oka and K. Waku, Prostaglandins Leukotrienes and Essential Fatty Acids, 2002, 66, 173-192.
2. H. H. O. Schmid, Chemistry and Physics of Lipids, 2000, 108, 71-87.
3. M. J. O'Neil, The Merck Index Thirteenth Edition, 2001, p105

