It07:Ammonia
| It07:Ammonia | |
|---|---|
| |
| General | |
| Systematic name | Azane NH3 |
| Molecular formula | NH3 |
| SMILES | [H]N([H])[H] |
| CAS number | 7664-41-7 |
| PubChem Identifier | 222 |
| Properties | |
| Molar mass | 241.29 gmol-1 |
| Melting point | 230-231oC |
| Boiling Point | 398.8 +/- 30.0oC |
| Density | 0.6942 gcm-3 |
| Solubility in water | 89.9g per 100 ml |
Introduction
Ammonia is a colourless gas at room temperature which has a smell that quite literally blows your head off! However, Ammonia is an extremely important molecule used in many reactions. Ammonia is made from hydrogen and nitrogen in the Haber process.
Molecular Structure
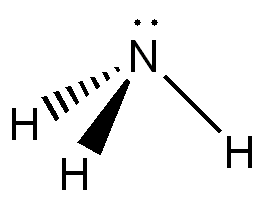
Ammonia has a trigonal pyramidal structure, consisting of central nitrogen atom and 3 hydrogen atoms resulting in C3V symmetry. The nitrogen atom has a lone pair, which using VSEPR theory explains the bent structure (lone pair – bonding pair repulsion). This structure results in a permanent overall dipole moment and thus is polar. In addition the easy formation of hydrogen bonds allow pure ammonia to be turned into liquid easily at -33.3°C and turned into a solid at -77.7°C. Ammonia undergoes inversion of the hydrogen atoms around the central nitrogen atom. The energy required is 24.7 kJ/mol, the frequency of this inversion at room temperature is 23.79 GHz.
MO of Ammonia
3D Structure
Hyoscine |
Uses
Ammonia is a nucleophile, using its lone pair to attack electron deficient areas usually resulting in a substitution. Reacting ammonia with molecules with good leaving groups such as alkyl halides results in an amine product for example; Methylamine is prepared commercially by the reaction of ammonia with chloromethane. Ammonia reacts with esters, acyl chlorides and anhydrides to give amide products.
The lone pair in ammonia makes it a good ligand. When bound as a ligand it is called an ammine ligand. It is a σ-donor ligand and is found coordinated to many transition metal centres. The hydrogens on the ammine ligand become more acidic when the ligand is bound.
Ammonia is used in the formation of nitric acid, this is the largest use of ammonia because nitric acid is used to make fertilizers as well as explosives. In this process ammonia is passed over a platinum catalyst at high temperatures in the presence of oxygen, oxidising it to nitric acid.

