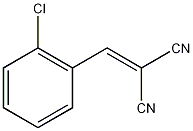
It07:2 – Chlorobenzalmalononitrile
| It07:2 – Chlorobenzalmalononitrile | |
|---|---|
| |
| General | |
| Systematic name | 2 - Chlorobenzalmalononitrile |
| Other names | CS Gas, Tear Gas |
| Molecular formula | C10H5ClN2 |
| Molar mass | 188.6 |
| Appearance | White crystal powder |
| CAS number | 2698-41-1 |
| Properties | |
| Density & phase | 1.04 g/cm³ |
| Melting point | 366 K at |
| Boiling point | 583 K |
History
First discovered by Ben Corson and Rodger Straughton in 1928. It was then developed in England in the 1950s where it was tested on animals to start with and then on volunteers from the British army. The testing on the animals did not show much because it has little to no effect on them because of their fur and under developed tear ducts.
Synthesis
CS is manufactured through the synthesis of 2 – chlorobenzaldehyde and molononitrile. The reaction is called the Knoevenagel Condensation reaction.
A weak base such as pyridine catalyses the reaction. The solid is harmless if wet however when dry the effects can be felt. The main dispersal method of the “defensive measure” solid is in aerosol form.
Effects
The effects of the gas form of the solid can vary from tearing of the eyes to server vomiting and prostration. CS gas is the less toxic form of “crowd control” gases and so is widely used around the world. The effects on people vary by the way the gas is delivered. In gas / aerosol form the particles react with the moisture on the skin and in the eyes. This causes a feeling of burning on the skin and in the eyes and so people’s eyes shut involuntarily. In some cases, the burning sensation can be felt in the nose and throat. It can also cause dizziness and vomiting.
Long Term Effects
CS gas is suppose to be non-lethal (a reason why it is widely spread) however, it can cause pulmonary damage and cause harm to the heart and liver.
On the 28th September 2000 Prof. Dr. Uwe Heinrich released a study commissioned by John Danforth, a member of “The Office of Special Counsel”. He concluded it depended on two factors:
1 Gas Mask
2 Closed within a room
He realised that if a gas mask was not being used and you were enclosed in a room then the effects described above can be possible[1]
Distribution
CS gas is used all over the world. The widely spread dispersal method is through blank pistol cartridges that contain dry CS powder.
Countermeasures
To remove CS gas from clothes, wash them in an alkaline solution of water with 5% sodium bisulfite. To stop eyes from hurt pour cow’s milk into them. This will stop the pain however; the burning sensation on the skin and inside the throat and breathing difficulties will persist.
