Irb10organicmodule
Synthetic Modelling Lab
Part one
The Hydrogenation of Cyclopentadiene Dimer

In this first part of the module, we use a molecular mechanics approach to determine whether the cyclodimerisation of cyclopentadiene and the hydrogenation of the dimer are kinetically or thermodynamically controlled. Dimer 1 is the exo dimer. Dimer 2 is the endo dimer. Dimers 3 and 4 are the two possible dihydro derivatives. Dimer five is the tetrahydro derivative. The geometries of the five dimers were optimised and the energies calculated in Avogadro. The calculation, using the MMFF94 force field, yields values of energy in kcal/mol. The results obtained are reported below:
TOTAL BOND STRETCHING ENERGY = 3.53954 kcal/mol
TOTAL ANGLE BENDING ENERGY = 30.78464 kcal/mol
TOTAL STRETCH BENDING ENERGY = -2.04223 kcal/mol
TOTAL TORSIONAL ENERGY = -2.69901 kcal/mol
TOTAL OUT-OF-PLANE BENDING ENERGY = 0.01642 kcal/mol
TOTAL VAN DER WAALS ENERGY = 12.76773 kcal/mol
TOTAL ELECTROSTATIC ENERGY = 13.01273 kcal/mol
TOTAL ENERGY = 55.37983 kcal/mol
TOTAL BOND STRETCHING ENERGY = 3.50402 kcal/mol
TOTAL ANGLE BENDING ENERGY = 33.45098 kcal/mol
TOTAL STRETCH BENDING ENERGY = -2.09834 kcal/mol
TOTAL TORSIONAL ENERGY = -3.13505 kcal/mol
TOTAL OUT-OF-PLANE BENDING ENERGY = 0.02180 kcal/mol
TOTAL VAN DER WAALS ENERGY = 12.52766 kcal/mol
TOTAL ELECTROSTATIC ENERGY = 14.30844 kcal/mol
TOTAL ENERGY = 58.57951 kcal/mol
The energy of dimer 2, the endo dimer, is higher than the energy of dimer 1, the exo dimer, by 3.2 kcal/mol. This result suggests that dimer 1, the exo dimer, is more stable than dimer 2, the endo dimer, in a thermodynamic sense: the exo dimer is the thermodynamic product. In practice, the endo dimer is the exclusive product of the reaction1. The dimerisation of cyclopentadiene is a pericyclic reaction. The regioselectivity and stereoselectivity of the reaction is controlled by the electronic properties of the molecules in the reaction. We can conclude that the dimerisation of cyclopentadiene is a kinetically controlled reaction.
TOTAL BOND STRETCHING ENERGY = 3.31099 kcal/mol
TOTAL ANGLE BENDING ENERGY = 31.93859 kcal/mol
TOTAL STRETCH BENDING ENERGY = -2.10220 kcal/mol
TOTAL TORSIONAL ENERGY = -1.47082 kcal/mol
TOTAL OUT-OF-PLANE BENDING ENERGY = 0.01327 kcal/mol
TOTAL VAN DER WAALS ENERGY = 13.63634 kcal/mol
TOTAL ELECTROSTATIC ENERGY = 5.11953 kcal/mol
TOTAL ENERGY = 50.44569 kcal/mol
TOTAL BOND STRETCHING ENERGY = 2.81434 kcal/mol
TOTAL ANGLE BENDING ENERGY = 24.76327 kcal/mol
TOTAL STRETCH BENDING ENERGY = -1.65266 kcal/mol
TOTAL TORSIONAL ENERGY = -0.33675 kcal/mol
TOTAL OUT-OF-PLANE BENDING ENERGY = 0.00049 kcal/mol
TOTAL VAN DER WAALS ENERGY = 10.55252 kcal/mol
TOTAL ELECTROSTATIC ENERGY = 5.14761 kcal/mol
TOTAL ENERGY = 41.28881 kcal/mol
The energy of dimer 3 is higher than the energy of dimer 4 by 9.2 kcal/mol. This result suggests that dimer 4 is more stable than dimer 3, in a thermodynamic sense. There is a greater difference in energy between dimers 3 and 4 than between the other pair of dimers, 1 and 2. Dimer 4 is the thermodynamic product.
Dimer five - Tetrahydro derivative:
TOTAL BOND STRETCHING ENERGY = 7.53384 kcal/mol
TOTAL ANGLE BENDING ENERGY = 33.23086 kcal/mol
TOTAL STRETCH BENDING ENERGY = -1.17442 kcal/mol
TOTAL TORSIONAL ENERGY = 2.22079 kcal/mol
TOTAL OUT-OF-PLANE BENDING ENERGY = 0.00000 kcal/mol
TOTAL VAN DER WAALS ENERGY = 8.54557 kcal/mol
TOTAL ELECTROSTATIC ENERGY = 0.00000 kcal/mol
TOTAL ENERGY = 50.35664 kcal/mol
The energy of dimer 5, the tetrahydro derivative, is higher than the energies of both dimers 3 and 4. This result is not conclusive, as only energies calculated for different isomers can be compared. Dimers 1 and 2, and 3 and 4, are isomers.
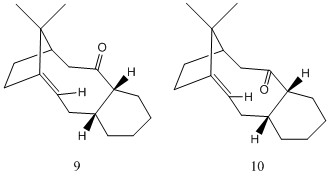
In this second exercise, we use a molecular mechanics approach, as in the first exercise, to determine which of the two isomers of a key intermediate in the synthesis of the cancer drug Taxol is the more stable. The two isomers differ in the directionality of the carbonyl group: the carbonyl group points up in intermediate 9, but point down in intermediate 10. Furthermore, the structure of the intermediate incorporates a cyclohexane ring, which can adopt a number of different conformations. We calculate the energy of the two isomers in Avogadro, taking into account the possibility of different conformations of the cyclohexane ring arising. The calculation, using the MMFF94s force field, yields values of energy in kcal/mol. The results are reported below:
Isomer 9
TOTAL BOND STRETCHING ENERGY = 7.93657 kcal/mol
TOTAL ANGLE BENDING ENERGY = 26.15260 kcal/mol
TOTAL STRETCH BENDING ENERGY = -0.11177 kcal/mol
TOTAL TORSIONAL ENERGY = 4.12210 kcal/mol
TOTAL OUT-OF-PLANE BENDING ENERGY = 1.77640 kcal/mol
TOTAL VAN DER WAALS ENERGY = 34.40240 kcal/mol
TOTAL ELECTROSTATIC ENERGY = 0.27176 kcal/mol
TOTAL ENERGY = 74.55007 kcal/mol
TOTAL BOND STRETCHING ENERGY = 8.21222 kcal/mol
TOTAL ANGLE BENDING ENERGY = 34.04482 kcal/mol
TOTAL STRETCH BENDING ENERGY = 0.07348 kcal/mol
TOTAL TORSIONAL ENERGY = 3.66209 kcal/mol
TOTAL OUT-OF-PLANE BENDING ENERGY = 1.65831 kcal/mol
TOTAL VAN DER WAALS ENERGY = 34.79887 kcal/mol
TOTAL ELECTROSTATIC ENERGY = 0.20042 kcal/mol
TOTAL ENERGY = 82.65022 kcal/mol
TOTAL BOND STRETCHING ENERGY = 8.27139 kcal/mol
TOTAL ANGLE BENDING ENERGY = 25.36965 kcal/mol
TOTAL STRETCH BENDING ENERGY = -0.04951 kcal/mol
TOTAL TORSIONAL ENERGY = 6.57367 kcal/mol
TOTAL OUT-OF-PLANE BENDING ENERGY = 1.53393 kcal/mol
TOTAL VAN DER WAALS ENERGY = 36.61481 kcal/mol
TOTAL ELECTROSTATIC ENERGY = 0.35652 kcal/mol
TOTAL ENERGY = 78.67047 kcal/mol
TOTAL BOND STRETCHING ENERGY = 8.32112 kcal/mol
TOTAL ANGLE BENDING ENERGY = 34.50343 kcal/mol
TOTAL STRETCH BENDING ENERGY = 0.20109 kcal/mol
TOTAL TORSIONAL ENERGY = 6.48706 kcal/mol
TOTAL OUT-OF-PLANE BENDING ENERGY = 1.78233 kcal/mol
TOTAL VAN DER WAALS ENERGY = 36.70603 kcal/mol
TOTAL ELECTROSTATIC ENERGY = 0.15645 kcal/mol
TOTAL ENERGY = 88.15751 kcal/mol
We find two chair conformers and two twist-boat conformers. Chair conformer 1 is lower in energy, by 8.1 kcal/mol, than chair conformer 2. This result suggests that the structure where the cyclohexane ring adopts a chair 1 conformation is more stable than that where it adopts a chair 2 conformation. Twist-boat conformer 1 is lower in energy, by 9.5 kcal/mol, than twist-boat conformer 2. Twist-boat conformer 1 is intermediate in energy between the two chair conformers, whereas twist-boat conformer 2 is the highest energy conformer of the four.
Isomer 10
TOTAL BOND STRETCHING ENERGY = 7.59435 kcal/mol
TOTAL ANGLE BENDING ENERGY = 18.80639 kcal/mol
TOTAL STRETCH BENDING ENERGY = -0.14264 kcal/mol
TOTAL TORSIONAL ENERGY = 0.23631 kcal/mol
TOTAL OUT-OF-PLANE BENDING ENERGY = 0.84557 kcal/mol
TOTAL VAN DER WAALS ENERGY = 33.26436 kcal/mol
TOTAL ELECTROSTATIC ENERGY = -0.05395 kcal/mol
TOTAL ENERGY = 60.55040 kcal/mol
TOTAL BOND STRETCHING ENERGY = 7.69183 kcal/mol
TOTAL ANGLE BENDING ENERGY = 16.88712 kcal/mol
TOTAL STRETCH BENDING ENERGY = -0.23245 kcal/mol
TOTAL TORSIONAL ENERGY = 2.08756 kcal/mol
TOTAL OUT-OF-PLANE BENDING ENERGY = 0.83283 kcal/mol
TOTAL VAN DER WAALS ENERGY = 33.98369 kcal/mol
TOTAL ELECTROSTATIC ENERGY = -0.06386 kcal/mol
TOTAL ENERGY = 61.18672 kcal/mol
3) Twist-boat conformation one:
TOTAL BOND STRETCHING ENERGY = 7.75750 kcal/mol
TOTAL ANGLE BENDING ENERGY = 19.01979 kcal/mol
TOTAL STRETCH BENDING ENERGY = -0.13154 kcal/mol
TOTAL TORSIONAL ENERGY = 3.72376 kcal/mol
TOTAL OUT-OF-PLANE BENDING ENERGY = 0.95208 kcal/mol
TOTAL VAN DER WAALS ENERGY = 35.02772 kcal/mol
TOTAL ELECTROSTATIC ENERGY = -0.06314 kcal/mol
TOTAL ENERGY = 66.28616 kcal/mol
4) Twist-boat conformation two:
TOTAL BOND STRETCHING ENERGY = 7.67614 kcal/mol
TOTAL ANGLE BENDING ENERGY = 17.65366 kcal/mol
TOTAL STRETCH BENDING ENERGY = -0.02562 kcal/mol
TOTAL TORSIONAL ENERGY = 4.41667 kcal/mol
TOTAL OUT-OF-PLANE BENDING ENERGY = 0.65873 kcal/mol
TOTAL VAN DER WAALS ENERGY = 35.16445 kcal/mol
TOTAL ELECTROSTATIC ENERGY = 0.14140 kcal/mol
TOTAL ENERGY = 65.68543 kcal/mol
We find two chair conformers and two twist-boat conformers. Chair conformer 1 is lower in energy, by 0.6 kcal/mol, than chair conformer 2. Twist-boat conformer 2 has a lower energy, by 0.6 kcal/mol, than twist-boat conformer 1, in contrast with isomer 9. Both twist-boat conformers are higher in energy than the chair conformers, again, in contrast with isomer 9, where one of the twist-boat conformers was intermediate in energy between the chair conformers. The results suggest that isomer 10 is more stable than isomer 9. All conformers of isomer 10 are lower in energy than the energy of the lowest-energy conformer of isomer 9. The difference in energy between the highest and the lowest energy conformers, for each isomer, is reported below:
ΔE(Isomer 9) = 13.61 kcal/mol
ΔE(Isomer 10) = 5.74 kcal/mol
The difference between the average energy of isomer 9 conformers and the average energy of isomer 10 conformers is reported below:
ΔE = 17.94 kcal/mol
We notice that the above value, the difference between the average energies of isomer 9 and isomer 10 conformers, is greater than the ranges of variation in conformer energy for both isomers. This result confirms that the directionality of the carbonyl group is a very important factor in determining the energy of the two isomers, even more important than the conformation which the cyclohexane ring adopts. We conclude that isomer 10 is the most stable isomer.
As mentioned before, isomers 9 and 10 are intermediates in the synthesis of the cancer drug Taxol. In the subsequent steps, functionalisation of the alkene is slow. This is because the carbon-carbon double bond lies adjacent to a bridgehead, which provides extensive stabilisation.
Spectroscopic Simulation using Quantum Mechanics

In this third exercise, we run a spectroscopic simulation of a second intermediate in the synthesis of Taxol, molecule 18, a derivative of isomer 10, which was analysed in the previous part. In the first step, the molecule is drawn and its geometry optimised in Avogadro, using the MMFF94s force field. In the second step, a further geometry optimisation is run, this time quantum mechanical in nature, at the density functional theory (DFT) level, and including the simulation of the 13C and 1H NMR spectra. The quantum mechanical calculation in question is invoked by the following keywords:
#B3LYP/6-31G(d,p) Opt SCRF=(CPCM,Solvent=benzene) Freq NMR EmpiricalDispersion=GD3
The results are reported below:
Geometry Optimisation
TOTAL BOND STRETCHING ENERGY = 24.53851 kcal/mol
TOTAL ANGLE BENDING ENERGY = 51.62600 kcal/mol
TOTAL STRETCH BENDING ENERGY = -0.46569 kcal/mol
TOTAL TORSIONAL ENERGY = 35.74268 kcal/mol
TOTAL VAN DER WAALS ENERGY = 67.15082 kcal/mol
TOTAL ELECTROSTATIC ENERGY = -4.71288 kcal/mol
TOTAL ENERGY = 178.08680 kcal/mol
NMR Calculation
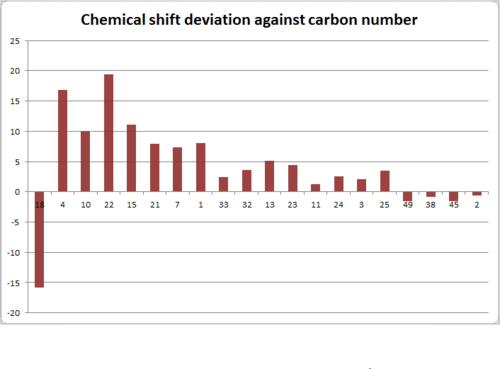
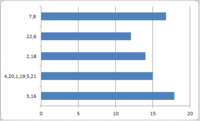
The results of the NMR simulation are summarised below. The graph shows the difference between the calculated chemical shifts and the chemical shifts reported in the literature2.
Vibrational Analysis
Sum of electronic and thermal Free Energies: -1651.359182
Part two - Analysis of the properties of the synthesised alkene epoxides
In this second part of the module, we analyse the properties of two epoxides, trans-stilbene and 1,2-Dihydronaphthalene. We will synthesise these two epoxides next week in the laboratory. Two catalysts will be used in the synthesis of the two chosen epoxides, the Shi catalyst and the Jacobsen catalyst. In the first section we analyse the crystal structures of species 21 and species 23, the stable precursors to the Shi catalyst and Jacobsen catalyst respectively, as retrieved from the Cambridge Crystallographic Data Centre (CCDC). In the second section, we locate and run a geometry optimisation on the two stable configurations of each epoxide on Avogadro, using the techniques learned in part one. We proceed to run an NMR calculation on the lowest-energy configuration of each epoxide. In the third section, we calculate a number of chiroptical properties, most importantly, the optical rotation. In the fourth section, we study the non-covalent interactions and electronic topology in the active site of one transition state. We also, finally, propose and analyse a new candidate epoxide for computational investigation of its chiroptical properties, as found by searching in the literature.
Crystal Structures
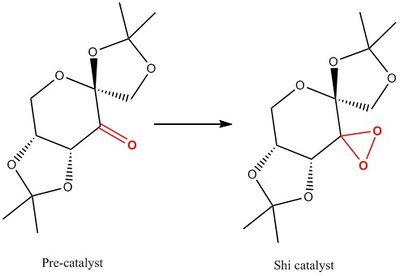
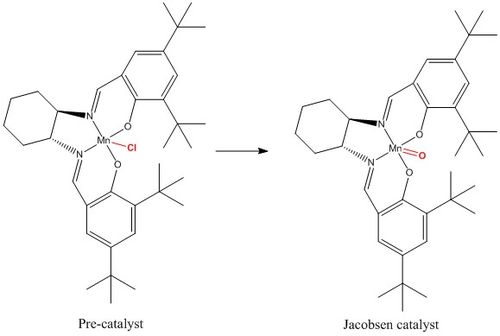
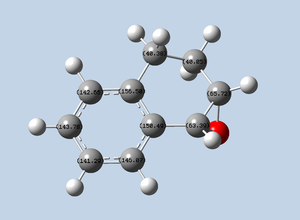
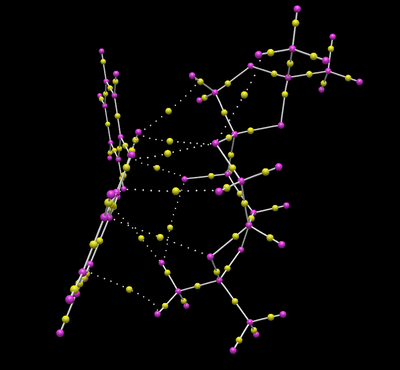
In this section we study the crystal structures of the Shi and Jacobsen pre-catalysts. The image below shows the chemical structures of both pre-catalyst and active catalyst:
Shi pre-catalyst
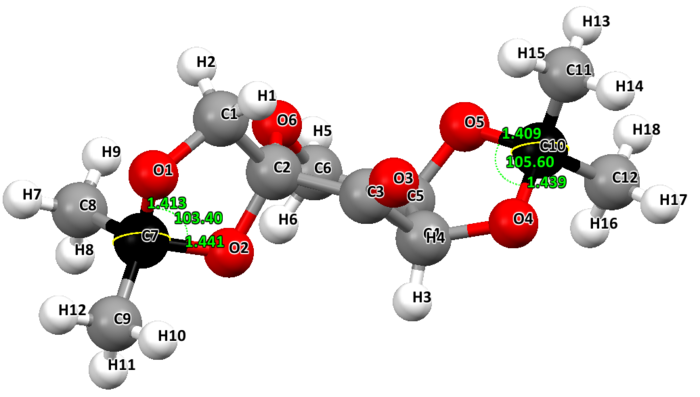
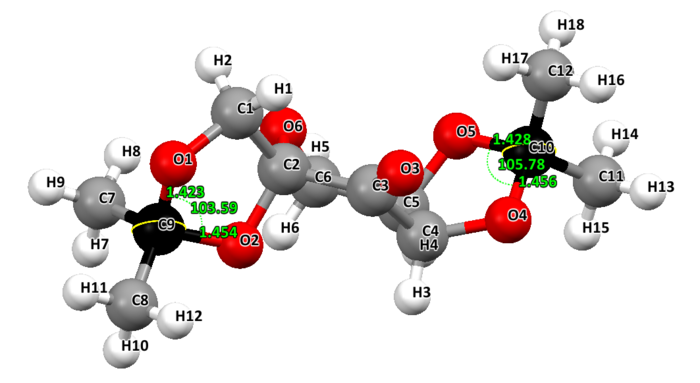
Two crystal structures are found on the Cambridge Crystallographic Data Centre, NELQEA and NELQEA01. They are both a perfect match of the pre-catalyst structure. The analysis is succinctly reported in the form of an image file; two anomeric centres are present in the molecule. These are labelled in black. Most important C-O bond lengths and O-C-O angles, centred on the two anomeric centres, are reported.
NELQEA:
NELQEA01:
Jacobsen pre-catalyst
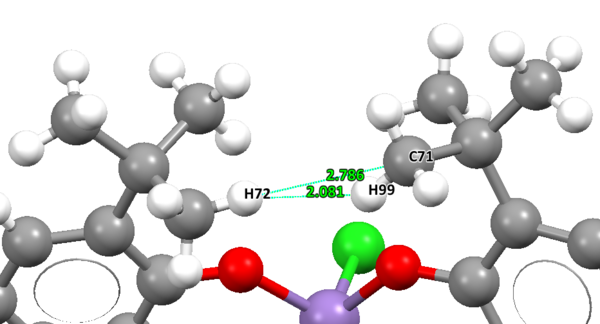
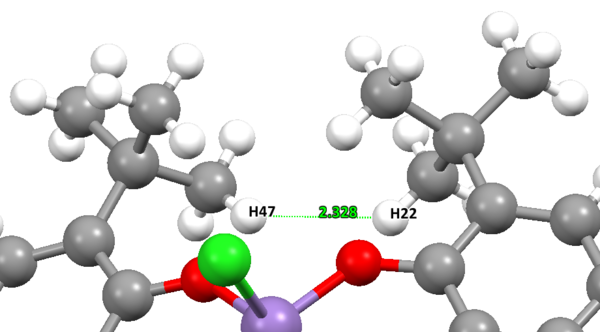
Five crystal structures are found on the Cambridge Crystallographic Data Centre, of which three are a perfect match of the pre-catalyst structure, but only two functional, TOVNIB01 and TOVNIB02. No anomeric centres are present in the molecule. The important structural feature here is the presence of tertiary butyl substituents on the benzene rings adjacent to the catalyst active site. We can see clearly from both crystal structures that the tertiary butyl groups closest to the active site interact with each other. TOVNIB01 has two short contacts, where the distance between the interacting atoms is less than the sum of their Van der Waals radii, whereas TOVNIB02 has one short contact. In both structures there is an interaction between two H atoms on adjacent tertiary butyl groups, in TOVNIB01 there is an additional interaction between an H atom and a C atom on adjacent tertiary butyl groups. The existence of these interactions is probably significant and important in determining the structure and energy of the transition state in the epoxidation reaction.
TOVNIB01
TOVNIB02
Geometry Optimisation
Trans-stilbene
Configuration one
TOTAL BOND STRETCHING ENERGY = 3.72551 kcal/mol
TOTAL ANGLE BENDING ENERGY = 2.69242 kcal/mol
TOTAL STRETCH BENDING ENERGY = -1.54161 kcal/mol
TOTAL TORSIONAL ENERGY = 2.33061 kcal/mol
TOTAL OUT-OF-PLANE BENDING ENERGY = 0.00296 kcal/mol
TOTAL VAN DER WAALS ENERGY = 26.86954 kcal/mol
TOTAL ELECTROSTATIC ENERGY = 5.37765 kcal/mol
TOTAL ENERGY = 39.45707 kcal/mol
Configuration two
TOTAL BOND STRETCHING ENERGY = 3.72626 kcal/mol
TOTAL ANGLE BENDING ENERGY = 2.69354 kcal/mol
TOTAL STRETCH BENDING ENERGY = -1.54201 kcal/mol
TOTAL TORSIONAL ENERGY = 2.33077 kcal/mol
TOTAL OUT-OF-PLANE BENDING ENERGY = 0.00294 kcal/mol
TOTAL VAN DER WAALS ENERGY = 26.87585 kcal/mol
TOTAL ELECTROSTATIC ENERGY = 5.36984 kcal/mol
TOTAL ENERGY = 39.45719 kcal/mol
1,2-dihydronaphthalene
Configuration one
TOTAL BOND STRETCHING ENERGY = 2.69756 kcal/mol
TOTAL ANGLE BENDING ENERGY = 2.18149 kcal/mol
TOTAL STRETCH BENDING ENERGY = -0.86887 kcal/mol
TOTAL TORSIONAL ENERGY = 1.67500 kcal/mol
TOTAL OUT-OF-PLANE BENDING ENERGY = 0.00950 kcal/mol
TOTAL VAN DER WAALS ENERGY = 19.37639 kcal/mol
TOTAL ELECTROSTATIC ENERGY = 5.15336 kcal/mol
TOTAL ENERGY = 30.22442 kcal/mol
Configuration two
TOTAL BOND STRETCHING ENERGY = 2.77950 kcal/mol
TOTAL ANGLE BENDING ENERGY = 2.62599 kcal/mol
TOTAL STRETCH BENDING ENERGY = -0.80225 kcal/mol0
TOTAL TORSIONAL ENERGY = 1.01537 kcal/mol
TOTAL OUT-OF-PLANE BENDING ENERGY = 0.03058 kcal/mol
TOTAL VAN DER WAALS ENERGY = 19.95779 kcal/mol
TOTAL ELECTROSTATIC ENERGY = 5.07650 kcal/mol
TOTAL ENERGY = 30.68347 kcal/mol
NMR Calculation
Trans-stilbene
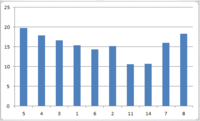
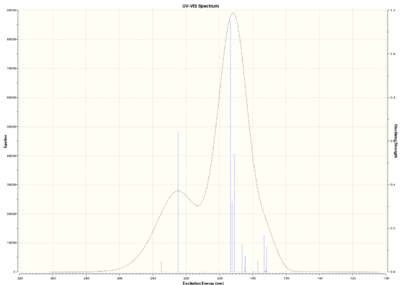
Above is a graph detailing the deviation of calculated 13C NMR chemical shifts from literature values3. We can see that the deviation is substantial. Trans-stilbene is an aromatic compound, with some carbon atoms lying in the same chemical environment. Thus, all chemical shifts are associated with more than one carbon atom; in particular, the shift at 143.6 ppm is associated with six carbon atoms. The NMR degeneracy tolerance was changed to 0.8 in order to 'focus' the NMR.
1,2-dihydronaphthalene
Above is a graph detailing the deviation of calculated 13C NMR chemical shifts from literature values4. We can see that, very much like reported earlier for trans-stilbene, the deviation is significant.
Assigning the absolute configuration
In this section, our objective is to calculate the chiroptical properties of the two epoxides that we will synthesise in the laboratory next week. The three properties which we can calculate here are the Optical Rotatory Power (ORP), the Electronic Circular Dichroism (ECD), and the Vibrational Circular Dichroism (VCD). Only the first property, the Optical Rotatory Power, can be readily measured in the third-year laboratory; we will proceed to compare the values obtained experimentally with the values obtained by computation.
Optical Rotation
The Optical Rotation is the only chiroptical property we can measure in the laboratory. The calculated optical rotatory power values of the two configurations of our two epoxides are reported below. We can see that the values for the two configurations of trans-stilbene are closely matched in absolute value and opposite in sign, as is expected. The absolute value for trans-stilbene is greater than 100; we can assume that this is a reliable prediction. On the other hand, the values for the two configurations of 1,2-dihydronaphthalene are not closely matched in absolute value and a change of sign is not observed between the two! The absolute value is lower than 50; this is not a reliable prediction.
Trans-stilbene
[Alpha] ( 5890.0 A) = 297.94 deg
[Alpha] ( 5890.0 A) = -287.25 deg
Literature values are reported below:
[Alpha] ( 5890.0 A) = 250.8 deg (22 °C)5
[Alpha] ( 5890.0 A) = 239.2 deg6
The literature values are comparable to our predicted values. The solvent used was chloroform. The deviation of our results from literature values are small.
1,2-Dihydronaphthalene
[Alpha] ( 5890.0 A) = -43.39 deg
[Alpha] ( 5890.0 A) = -155.82 deg
Literature values are reported below:
[Alpha] ( 5890.0 A) = -129 deg (20 °C)7
[Alpha] ( 5890.0 A) = -133 deg8
Looking at our calculated values, we can see that there is a good match, in absolute value and sign, between literature values and the calculated Optical Rotatory Power of one of the 1,2-dihydronaphthalene configurations. This is good news. The bad news is that the calculated value for the other configuration, the one expected to be positive in sign, is not only negative, but also differs in absolute value by nearly 150 deg from the value of the other configuration. This result suggests that there was an issue with the calculation leading to the former value.
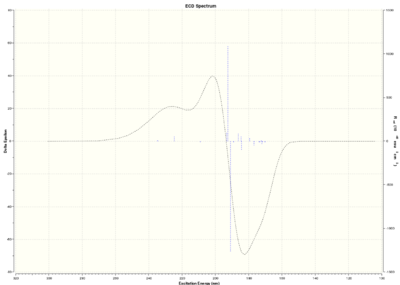
Electronic Circular Dichroism
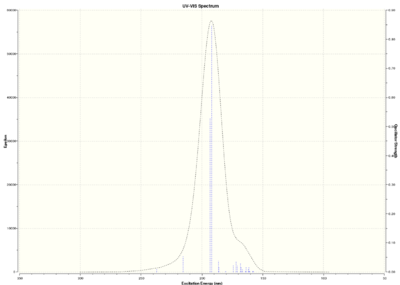
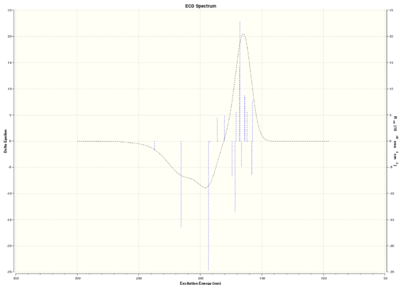
Optically active chiral molecules absorb circularly polarised light in a way which makes the measurement of Circular Dichroism spectra a useful technique to determine the absolute configuration of a molecule. If UV-Visible light is used, we talk about Electronic Circular Dichroism (ECD); where infrared light is used, we talk about Vibrational Circular Dichroism (VCD). Although ECD is not useful for epoxides because of the lack of a suitable chromophore, both ECD and VCD spectra are predicted in this section.
Trans-stilbene
Dihydronaphthalene
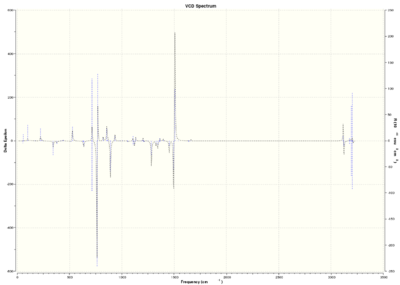
Vibrational Circular Dichroism
Trans-stilbene
Dihydronaphthalene
cis-β-methyl styrene
The purpose of this section is to prove that calculated thermochemical data can be used to obtain a value for the enantiomeric excess of the synthesised epoxide. Cis-β-methyl styrene is the epoxide used for the calculation as data is not available for our two epoxides. We pick the lowest-energy transition state of the (R,S)-epoxide and the lowest-energy transition state of the (S,R)-epoxide. We assume an equilibrium is set up between them, in such a way that permits us to obtain the value of the equilibrium constant, K, from the difference between the free energies of the two transition states.
Sum of electronic and thermal free energies = -3383.259559 (R,S)
Sum of electronic and thermal free energies = -3383.251060 (S,R)
Difference = 0.008499 = 22314.1262 J/mol (S,R)-(R,S)
K = 0.000123173
Enantiomeric excess = 99% (R,S)
Analysis of the active site of the transition state
The active site of one of the transition states in the Shi epoxidation of trans-stilbene is analysed in this sections, using two different approaches, the first examines exclusively the non-covalent interactions present and observable between substrate and catalyst, whereas the second examines both non-covalent and covalent interactions.
Investigating the non-covalent interactions
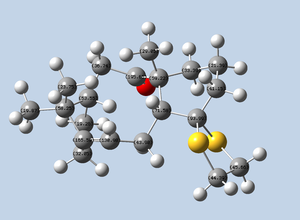
Trans-stilbene transition state number 5 (http://dx.doi.org/10.6084/m9.figshare.829524) was subjected to NCI analysis. Non-covalent interactions are dispersion interactions or hydrogen bonds, for example. These can be both attractive and repulsive. The result of NCI analysis is reported below in a rotatable format. The colours indicate whether interactions are attractive or repulsive. We see that the great majority of the interactions are mildly attractive, as green colour predominates, while repulsive interactions are particularly evident and localised in the active site, as shown in red.
Orbital |
Investigating the Electronic Topology
The same transition state for which NCI analysis is carried out is subjected to QTAIM analysis. The dynamism of bond breaking and formation are evident. We can see clearly the dioxirane in the process of forming new bonds with the alkene.
Suggesting new candidates for investigation
A search is conducted for an epoxide with a high absolute value of Optical Rotatory Power, above 500. The search results in a number of candidates, of which most possess additional chiral centres in addition to the epoxide. These are ruled out. One candidate is ruled out because it has the same structure as trans-stilbene, except for a methyl substitutent on one of the benzene rings. The chosen candidate has alkyne and alkene functionalities in addition to the epoxide. The optical rotation of two configurations of this candidate molecule was calculated. The results are reported below:
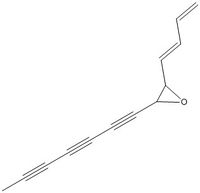
Configuration one: [Alpha] ( 5780.0 A) = 1063.69 deg
Configuration two: [Alpha] ( 5780.0 A) = 17.74 deg
Literature values are reported below, for a single configuration at two different wavelengths of light:
[Alpha] ( 5780.0 A) = -264 deg(20°)9
[Alpha] ( 3650.0 A) = -718 deg(20°)9
The results of this exercise are not conclusive. Both calculated optical rotations have a positive sign, in contrast with literature values. It cannot be ruled out that literature assignments are wrong. Further studies are needed to get to the bottom of this problem.
References
1. Pierluigi Caramella,* Paolo Quadrelli, and Lucio Toma, 'An Unexpected Bispericyclic Transition Structure Leading to 4+2 and 2+4 Cycloadducts in the Endo Dimerization of Cyclopentadiene', J. Am. Chem. Soc., 2002, 124 (7), pp 1130–1131
2. L. Paquette, N. A. Pegg, D. Toops, G. D. Maynard, R. D. Rogers, J. Am. Chem. Soc., 1990, 112, 277-283
3. Omar Boutureira, Joanna F. McGouran, Robert L. Stafford, Daniel P. G. Emmerson and Benjamin G. Davis, 'Accessible sugars as asymmetric olefin epoxidation organocatalysts: glucosaminide ketones in the synthesis of terminal epoxides', Org. Biomol. Chem., 2009, 7, Supplementary Info
4. Keith Smith, Chia-Hui Liu and Gamal A. El-Hiti, 'A novel supported Katsuki-type (salen)Mn complex for asymmetric epoxidation', Org. Biomol. Chem., 2006, 004, 926
5. Fox, David J.; Pedersen, Daniel Sejer; Petersen, Asger B.; Warren, Stuart, Organic and Biomolecular Chemistry, 2006, vol. 4, #16 p. 3117-3119
6. Denmark, Scott E.; Matsuhashi, Hayao, Journal of Organic Chemistry, 2002, vol. 67, #10 p. 3479-3486
7. Pedragosa-Moreau, S.; Archelas, A.; Furstoss, R., Tetrahedron, 1996, vol. 52, #13 p. 4593-4606
8. Boyd, Derek R.; Sharma, Narain D.; Agarwal, Rajiv; Kerley, Nuala A.; McMordie, R. Austin S.; et al., Journal of the Chemical Society, Chemical Communications, 1994, #14 p. 1693-1694
9. Bohlmann, F. et al. Chemische Berichte, 1962, vol. 95, p. 1320-1327