Inorgjwy17
Molecule Computational Analysis
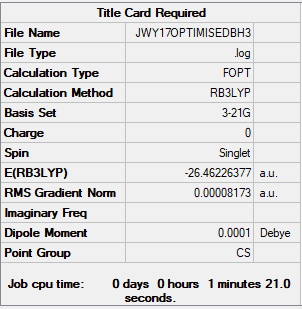
BH3
B3LYP/3-21G Method
Item Value Threshold Converged? Maximum Force 0.000206 0.000450 YES RMS Force 0.000107 0.000300 YES Maximum Displacement 0.001134 0.001800 YES RMS Displacement 0.000605 0.001200 YES
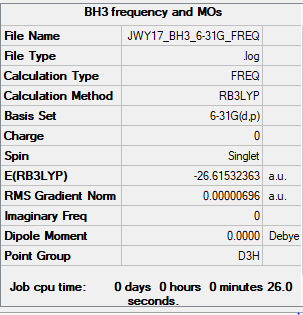
Frequency (B3LYP/3-21G)
Link to Frequency Analysis .log file B3LYP/3-21G BH3 Frequency
Low frequencies --- 0.0004 0.0005 0.0006 40.7057 42.0009 47.9484 Low frequencies --- 1146.2825 1204.7702 1204.93534.9353
BH3 |
| Mode | Frequency | Intensity (arbitrary unit) | IR active? | Type |
|---|---|---|---|---|
| 1 | 1146 | 92 | Yes | Out-of-plane Bend |
| 2 | 1205 | 12 | Slightly | Scissor (bend) |
| 3 | 1205 | 12 | Slightly | Scissor (bend) |
| 4 | 2591 | 0 | No | Symmetric Stretch |
| 5 | 2729 | 104 | Yes | Asymmetric Stretch |
| 6 | 2729 | 104 | Yes | Asymmetric Stretch |
The reasons for 3 peaks in the IR spectrum despite having 6 vibrational modes are:
1. The lack of dipole moment change in vibrational mode 4. This means it is not IR active and no peak for this particular vibration.
2. The degeneracy of mode 2,3 and 5,6. Their peaks would overlap and form a single peak. This explains, as well, why the peak at 2729cm-1 is almost double the height of the peak at 1146cm-1.
B3LYP/6-31G Method
Item Value Threshold Converged? Maximum Force 0.000014 0.000450 YES RMS Force 0.000007 0.000300 YES Maximum Displacement 0.000055 0.001800 YES RMS Displacement 0.000027 0.001200 YES
Frequency Analysis (B3LYP/6-31G)
Link to Frequency Analysis .log file B3LYP/6-31G BH3 Frequency
| Mode | Frequency | Intensity (arbitrary unit) | IR active? | Type |
|---|---|---|---|---|
| 1 | 1162 | 92 | Yes | Out-of-plane Bend |
| 2 | 1213 | 14 | Slightly | Scissor (bend) |
| 3 | 1213 | 14 | Slightly | Scissor (bend) |
| 4 | 2582 | 0 | No | Symmetric Stretch |
| 5 | 2715 | 126 | Yes | Asymmetric Stretch |
| 6 | 2715 | 126 | Yes | Asymmetric Stretch |
As shown, using different basis sets the vibrational frequency, intensity are slightly different. However, the type of vibration and IR activity of the vibrations remain the same. The IR spectrum therefore, will have peak at slightly different frequencies compared to the 3-21G method, possessing the same relative positions.
Good vibrational analysis although you are missing the symmetries of each mode from the table. In general, while nice to see the 3-21G calculation results they aren't really relevant to the report content and were included in the lab more for revision purposes. It would have been better to have made some comment maybe on why the calculation was done at the lower basis-set first to make it relevant. The IR spectrum should have been taken from the higher level calculation too, although your reasons above for the 3 visible peaks are correct and you have at least compared the two spectra here. Smf115 (talk) 14:07, 2 June 2019 (BST)
MO diagram and Orbitals
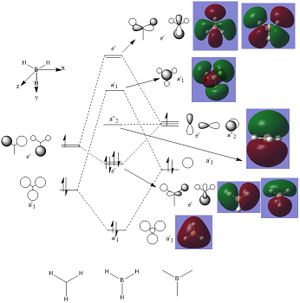
Good inclusion of the calculated MOs on to the MO diagram. To improve, you could have considered some of the subtler differences between the calculated MOs and the LCAO MOs, for example, consider the relative orbital contributions in the 3a1' or 2e' MOs. Smf115 (talk) 14:10, 2 June 2019 (BST)
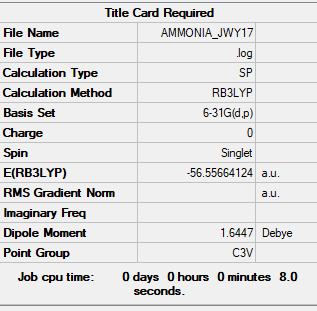
NH3 BH3 Association Energy
The heading is for NH3BH3 but the summary table is for NH3. Information should have been included for both molecules and you're missing the frequency log files for both which means there is no supporting evidence for your energies below. Smf115 (talk) 14:35, 2 June 2019 (BST)
E(NH3)= -56.5566 a.u.
E(BH3)= -26.6153 a.u.
E(NH3BH3)= -83.2247 a.u.
ΔE=E(NH3BH3)-[E(NH3)+E(BH3)] = -0.0528 a.u
= -138.95 kJ/Mol
Compared to ethane C-C bond (376.56kJ/Mol), the B-N bond is only about a third as strong, therefore the B-N bond is considered medium strong relative to the ethane C-C bond.
Correct calculation although it hasn't been written out and your final answer is a little out. I think this is because you've used rounded energy values for the calculation when you should use the full figures and then round the final reported energy value to the correct degree of accuracy (5 d.p. for a.u. and nearest 1 kJmol-1). Good comparison made to evaluate the strength - but always have references for literature values! Smf115 (talk) 14:31, 2 June 2019 (BST)
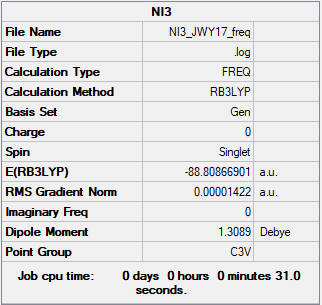
NI3
B3LYP/6-31G(d,p)LANL2DZ Method
Item Value Threshold Converged?
Maximum Force 0.000028 0.000450 YES
RMS Force 0.000014 0.000300 YES
Maximum Displacement 0.000242 0.001800 YES
RMS Displacement 0.000122 0.001200 YES
Frequency (B3LYP/6-31G(d,p)LANL2DZ)
Link to Frequency Analysis .log file NI3 Frequency
Low frequencies --- -0.0576 -0.0292 -0.0031 1.9682 2.0035 2.2189 Low frequencies --- 101.3080 101.3087 148.3159
NI3 |
N-I distance
The optimised molecule gave a N-I bond distance of 2.184 Angstrom.
Correct implementation of the pseudopotential and good structure information. Smf115 (talk) 14:39, 2 June 2019 (BST)
Mini-Project: Al2Cl4Br2
Isomers
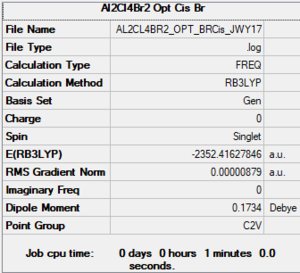
Cis - Al2C4Br2
Item Value Threshold Converged?
Maximum Force 0.000030 0.000450 YES
RMS Force 0.000010 0.000300 YES
Maximum Displacement 0.000393 0.001800 YES
RMS Displacement 0.000164 0.001200 YES
Link to Frequency Analysis .log file Cis-Al2C4Br2
Low frequencies --- -1.4145 -0.5292 -0.0017 -0.0003 0.0002 2.3603 Low frequencies --- 17.4891 51.1468 78.5185
Cis - AlCBr (Red = BR) |
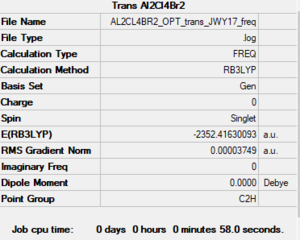
Trans - Al2C4Br2
Item Value Threshold Converged?
Maximum Force 0.000055 0.000450 YES
RMS Force 0.000020 0.000300 YES
Maximum Displacement 0.000295 0.001800 YES
RMS Displacement 0.000141 0.001200 YES
Link to Frequency Analysis .log file Trans-Al2C4Br2
Low frequencies --- -1.4581 -0.0018 0.0024 0.0026 0.8584 1.5599 Low frequencies --- 18.1956 49.1682 72.8886
Trans-Al2C4Br2 |
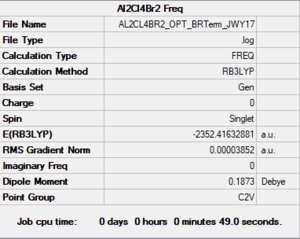
2Br Bridge-Al2C4Br2
Item Value Threshold Converged?
Maximum Force 0.000119 0.000450 YES
RMS Force 0.000044 0.000300 YES
Maximum Displacement 0.001376 0.001800 YES
RMS Displacement 0.000539 0.001200 YES
Link to Frequency Analysis .log file 2-Br Bridge-Al2C4Br2
Low frequencies --- -5.4581 -5.2150 -2.6435 0.0050 0.0050 0.0051 Low frequencies --- 14.7818 63.1287 85.9458
2Br Bridge-Al2C4Br2 |
Al2C4Br2 with terminal and bridging Br
The molecule is an enantiomer, which the other is the mirror image of the one shown. In a non chiral environment, they both posses the same vibrational modes and energy.
Item Value Threshold Converged?
Maximum Force 0.000049 0.000450 YES
RMS Force 0.000019 0.000300 YES
Maximum Displacement 0.001173 0.001800 YES
RMS Displacement 0.000344 0.001200 YES
Link to Frequency Analysis .log file 2-Br Bridge-Al2C4Br2
Low frequencies --- -1.2949 -0.0032 -0.0029 -0.0018 1.2691 1.6593 Low frequencies --- 16.9144 55.8709 80.0771
2-Br Bridge-Al2C4Br2 |
Al2C4Br2 with 2 Terminal Br
Item Value Threshold Converged?
Maximum Force 0.000050 0.000450 YES
RMS Force 0.000017 0.000300 YES
Maximum Displacement 0.001034 0.001800 YES
RMS Displacement 0.000357 0.001200 YES
Link to Frequency Analysis .log file 2-Br Bridge-Al2C4Br2
Low frequencies --- 0.0030 0.0035 0.0036 0.5786 0.9759 2.2139 Low frequencies --- 18.7071 51.2220 72.1983
2-Br Bridge-Al2C4Br2 |
Energy
E(trans-Al2Cl4Br2) = -2352.4163 a.u.
E(2-Br Bridge-Al2Cl4Br2) = -2352.4063 a.u.
ΔE = 0.0100 a.u.
= 26kJ/mol
Therefore, the trans-Al2Cl4Br2 is the more stable isomer of the 2. However, among all the isomers of the molecule, Al2Cl4Br2 with 2 terminal Br is the lowest in energy.
Impressive calculations of all isomers with the correct symmetries and pseudopotential input on all. Correct calculation here, to improve, it would have been nice to see some consideration given to explaining the relative stabilities. Smf115 (talk) 08:50, 31 May 2019 (BST)
Dissociation/Association Energy
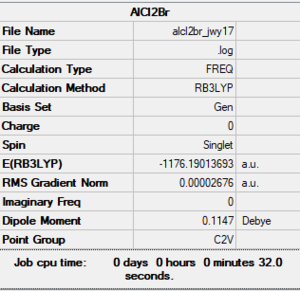
Trans-Al2Cl4Br2
E(AlCl2Br) = -1176.1901 a.u.
E(trans-Al2Cl4Br2) = -2352.4163 a.u.
ΔE = -2352.4163 -(-2 x 1176.1901)
= -0.0361 a.u.
= -95kJ/mol
The product is more stable than the isolated monomers, meaning the monomer is more likely to dimerise and form the product despite the drop in entropy. In the product, both Al-Cl terminal and Al-Cl bridge bond are longer than the Al-Cl bond in the monomer (2.094 Angstrom, 2.299 Angstrom, 2.089 Angstrom respectively). However, the 2 extra Al-Cl bonding in the product more than compensated for the increase in energy from the extended bond length.
Al2C4Br2 with 2 Terminal Br
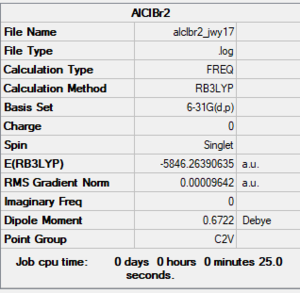
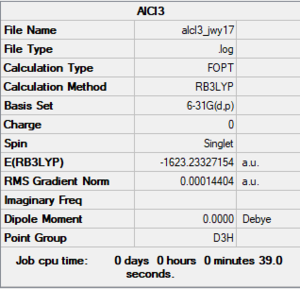
E(AlCl3) = -1623.2333 a.u.
E(AlClBr2) = -5846.2639 a.u.
E(Al2Cl4Br2 with 2 terminal Br) = - 2352.4163 a.u.
ΔE = - 2352.4163 - (-5846.2639 -1623.2333)
= 5117.0809 a.u.
= 1.35 x 10^4 kJ/mol
The isolated monomers are overwhelmingly more stable than the product. Based on the bond length changes, the Al-Br bond in the product is weaker than the monomer (Product: 2.277 Angstrom, AlClBr2 Monomer = 2.238 Angstrom). The Al-Cl bridge bond strength in the product is also significantly larger than the monomers (Al-Cl bridge: 2.301, AlClBr2: Al-Cl = 2.095 Angstrom, AlCl3: Al-Cl = 2.087 Angstrom). The longer bonds in the product molecule despite the 2 extra Al-Cl bridge bond seemed to increase the overall energy of the product.
Good calculations with the correct accuracy for the final energy values. Just note, you have calculated the association energy rather than the dissociation energy and haven't really identified this as the property that you're calculating. The extra analysis is really good to see. Sadly though, you've forgotten the pseudopotential on the AlCl3 and AlClBr2 monomers which is why there is such a massive energy difference when comparing to the dimer! You were also meant to submit the frequency log file for the AlCl2Br monomer and include the structure information for it. Smf115 (talk) 08:59, 31 May 2019 (BST)
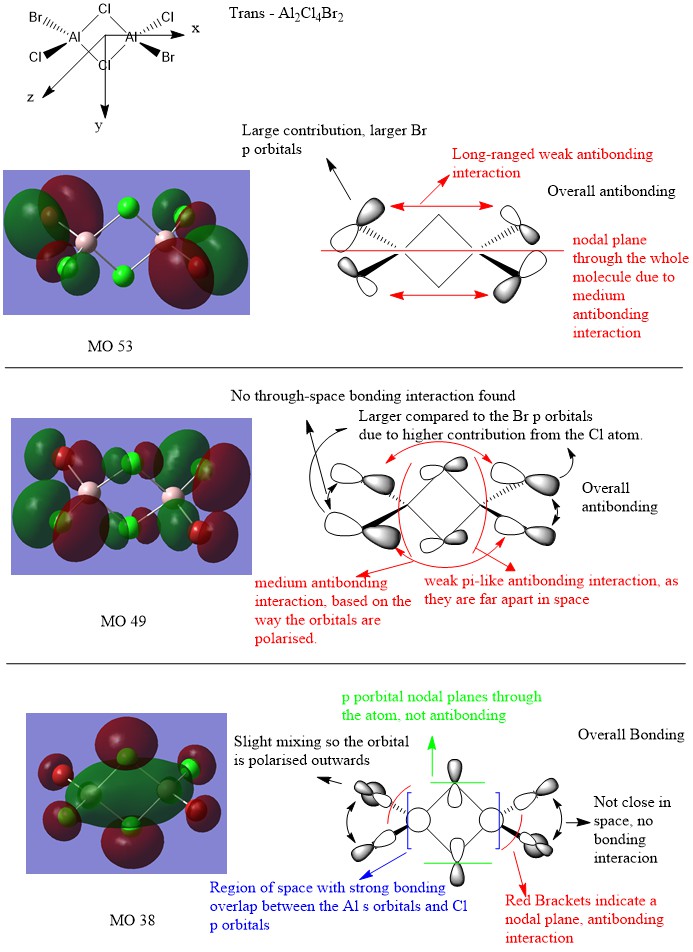
MO of trans-Al2Cl4Br2
You've chosen a good range of MOs to analyse and your annotations are clear with a good use of technical terms to explain the interactions. However, there are errors in some places (e.g. the main interaction in MO53 between the terminal and bridging orbitals hasn't been labelled). The LCAO for MO38 is incorrect (but it is a challenging one to tackle!), it might help to consider the diffuse nature of the Al orbitals and how the antibonding nature of the interaction of the Al orbital with the terminal ones might affect the shape of the terminal orbitals. Smf115 (talk) 09:10, 31 May 2019 (BST)