Inorganic comp3:tsa116
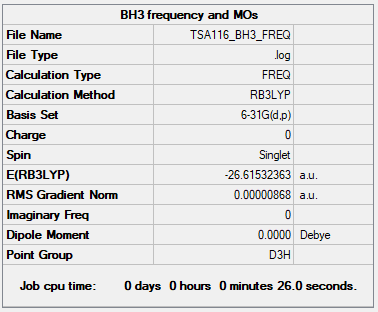
BH3
B3LYP/6-31G(d,p) level
Item Table
Item Value Threshold Converged? Maximum Force 0.000017 0.000450 YES RMS Force 0.000009 0.000300 YES Maximum Displacement 0.000068 0.001800 YES RMS Displacement 0.000034 0.001200 YES
Low Frequencies Table
Low frequencies --- -10.1111 -3.0903 -0.0054 0.4892 1.8274 3.5901 Low frequencies --- 1162.9534 1213.1537 1213.1564
Link to my frequency log file: click here.
BH3 |
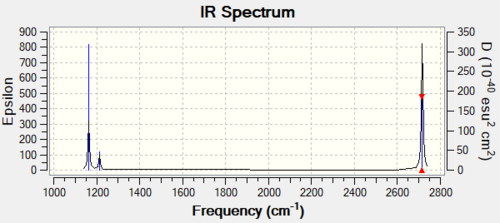
Vibrational Spectrum for BH3
| wavenumber (cm-1) | Intensity (arbitrary units) | symmetry | IR active? | type |
|---|---|---|---|---|
| 1163 | 93 | A2" | Yes | out-of-plane bend |
| 1213 | 14 | E' | Yes (low intensity) | in-plane scissoring |
| 1213 | 14 | E' | Yes (low intensity) | in-plane bending |
| 2582 | 0 | A1' | No | symmetric stretch |
| 2716 | 126 | E' | Yes | asymmetric stretch |
| 2716 | 126 | E' | Yes | asymmetric stretch |
There are only 3 peaks in the spectrum and yet 6 vibrational modes. This is because one vibrational mode is IR inactive (symmetric stretch) as there is no change in dipole moment; another reason is because for some vibrations (ie bends and stretches), they have the same symmetry label such as the E' stretches and E' bends. As a result, these will have the same frequency. Hence, we will only see 3 peaks corresponding to the E' stretch, E' bends, and the A2" bend.
Molecular Orbitals
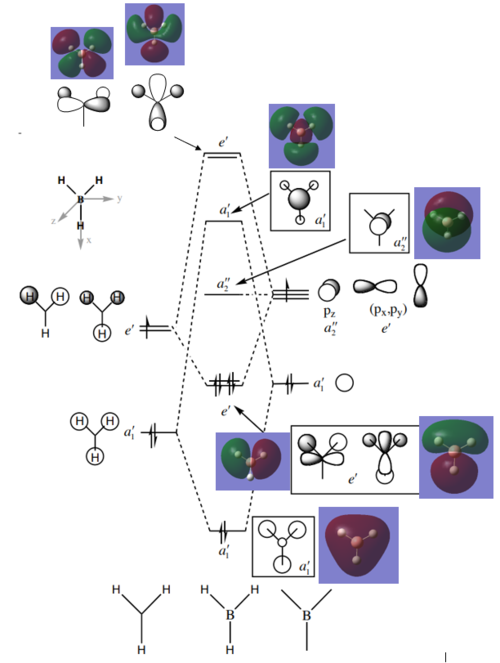
There is another orbital that is the lowest energy orbital (non-bonding). The Gaussian surface for this is as shown:
The original MO diagram (without the Gaussian calculated surfaces) was referenced from Hunt, P. Molecular Orbitals in Inorganic Chemistry Lecture 4, Imperial College London, London, 2018.
Are there significant differences? Generally, the overlap and phases are expected and there is no difference. However, the space that the MO occupies in some cases is significantly different, such as the e' antibonding MO at the top of the photo and the a1' antibonding orbital (where the s-orbitals seem to be bigger than the LCAO's prediction). The p-orbital of the B atom can be observed to have been bent downwards. While this is different from what the LCAO spatially predicts, it can be rationalised and is therefore not abnormal. In this case of BH3, the relative ordering of the MO energies are same in both the LCAO and Gaussian calculated models.
What does this say about the accuracy and usefulness of qualitative MO theory? This tells us that qualitative MO theory is a good predictor of the relative positioning, phases and overlap of the atomic orbitals. More importantly, qualitative MO theory is sufficient at this level of analysis (such as for small molecules and less complicated Mo systems). However, in reality the shape and the spatial arrangement of the MO is not the same as what can be achieved via qualitative MO theory. Therefore qualitative MO theory is limited in its usefulness, especially when the molecules get larger and more complicated.
Clear inclusion of the MOs in the diagram and great discussion of the similarities and the differences between the real and LCAO approaches. Smf115 (talk) 22:17, 13 May 2019 (BST)
NH3 and NH3BH3
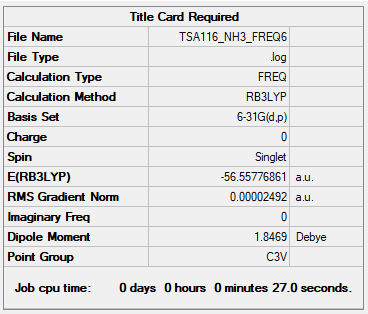
NH3
B3LYP/6-31G(d,p) level
Item Table
Item Value Threshold Converged? Maximum Force 0.000049 0.000450 YES RMS Force 0.000025 0.000300 YES Maximum Displacement 0.000206 0.001800 YES RMS Displacement 0.000122 0.001200 YES
Low Frequencies Table
Low frequencies --- -28.3213 -28.3065 -12.2798 -0.0035 0.0092 0.0587 Low frequencies --- 1089.9221 1694.1328 1694.1331
Link to my frequency log file: click here.
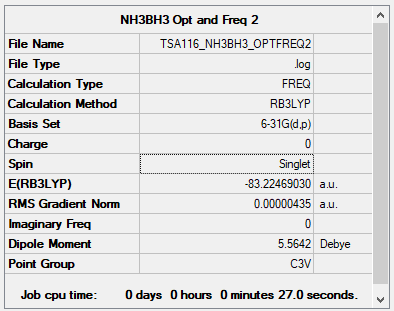
NH3BH3
B3LYP/6-31G(d,p) level
Item Table
Item Value Threshold Converged? Maximum Force 0.000007 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000048 0.001800 YES RMS Displacement 0.000022 0.001200 YES
Low Frequencies Table
Low frequencies --- -0.1729 -0.0774 -0.0067 12.6109 12.7247 12.7338 Low frequencies --- 263.3402 633.0319 639.1340
Link to my frequency log file: click here.
Association Energies
• E(NH3) = –56.55777 a.u.
• E(BH3) = –26.61532 a.u.
• E(NH3BH3) = –83.22469 a.u.
• ΔE = E(NH3BH3) – [E(NH3) + E(BH3)] = –0.05160 a.u. = –135 kJ mol–1
Based on literature values (Luo, Y. R., Comprehensive Handbook of Chemical Bond Energies, CRC Press, Boca Raton, Florida, 2007), the reported literature value of the N-B bond energy is 378 kJ mol–1. This tells us that the N-B dative bond present in NH3BH3 is relatively weak.
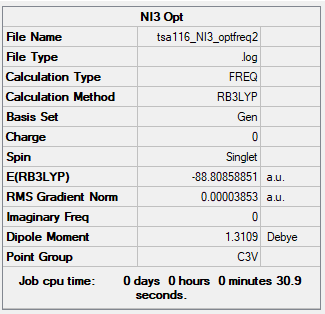
NI3
B3LYP/6-31G(d,p) LANL2DZ level
Item Table
Item Value Threshold Converged? Maximum Force 0.000068 0.000450 YES RMS Force 0.000044 0.000300 YES Maximum Displacement 0.000492 0.001800 YES RMS Displacement 0.000365 0.001200 YES
Low Frequencies Table
Low frequencies --- -12.7384 -12.7323 -6.2910 -0.0040 0.0188 0.0633 Low frequencies --- 101.0326 101.0333 147.4125
Link to my frequency log file: click here.
Jmol image
NI3 |
The optimised N-I bond distance is 2.184 Å.
Ionic Liquids
Charge Distribution
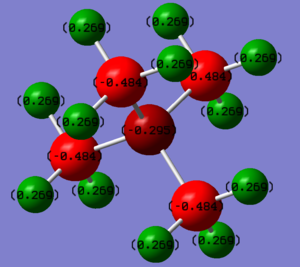

| Element/Atom | Charge on N(CH3)4 | Charge on P(CH3)4 |
|---|---|---|
| Hydrogen | 0.27 | 0.30 |
| Carbon | –0.48 | –1.06 |
| Central atom (N/P) | –0.30 | 1.67 |
The first main noticeable difference is the charge on the central atom. The N is negatively charged whereas the P is positively charged, suggesting a higher electron density around the N than the P. Additionally, this suggests that there is an excess of electron density around N, whereas there is an electron deficiency about P. This makes N-centre nucleophilic and the P-centre electrophilic. The electron density around the central atom can be rationalised by comparing the relative electronegativities of the central atom and Carbon:[1]
• Between N and C, N is more electronegative, therefore N will pull some electron density towards itself, making it be negatively charged
• Between P and C, C is more electronegative, therefore C will pull electron density away from P, making it positively charged
Therefore, even though a positive charge on the central atom is expected due to electron deficiency, the high electronegativity of N causes a positively charged N to be unstable, hence it pulls electrons towards itself to stabilise the molecule, hence causing the N atom to have a negative charge.
The charges on C and H are expected because C is slightly more electronegative than H, hence H will tend to have a positive charge. The charge on C is more negative in P(CH3)4 because N's higher electronegativity than P means that it pulls more of C's electron density away from itself. As such, more electron density from C is pulled away in N(CH3)4, making the charges on C less negative.
Discussion on the formal positive charge
The formal positive charge here is based on the electron deficiency of the central N atom using the Lewis structure theory. Because there are only 4 immediate electrons around the N atom when drawn in the way shown (which is drawn based on the Lewis structure theory), this is less than the 5 valence electrons that N has, hence it is 1 electron deficient and has a +1 charge.
However, based on our Gaussian calculated model, we know that the positive charge in the molecule actually resides on the terminal H atoms. Both the C and N atoms are all negatively charged.
Ok explanation of why the formal charge arises. The comparison of the electronegativities is good to justify the P and N charges but could be improved further by comparing values of the electronegativities from your references or considering symmetry for example. Smf115 (talk) 22:19, 13 May 2019 (BST)
Molecular Orbital visualisation
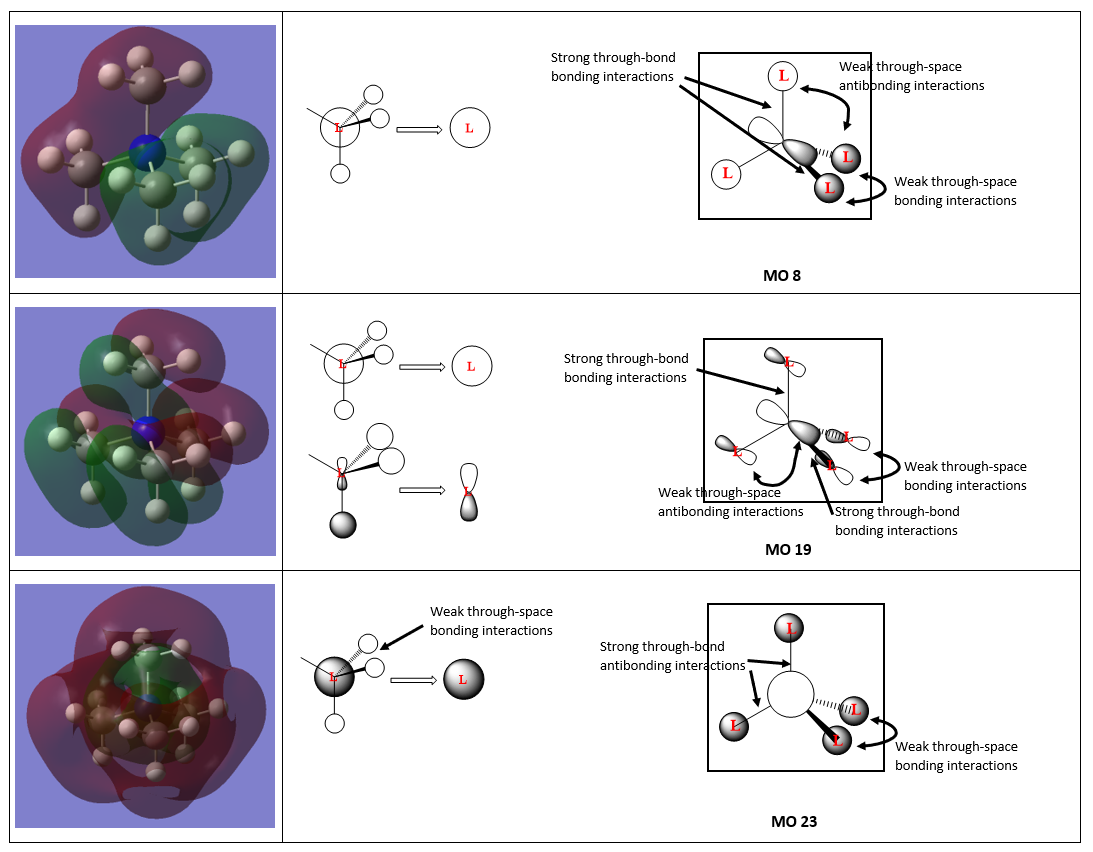
The ligand FOs are described for clarity:
• For MO 8, there is only one type of contributing ligand FO (since all the ligand FOs are the same), and they all form strong through-bond bonding interactions with the central N atom. The ligand FO is formed from the a1' bonding fragment orbital which has all the atomic orbitals of the atoms in the same phase. There is a weak through-space bonding interaction between the ligand FOs of the same phase, and weak through-space antibonding interaction between ligand FOs of the opposite phase. The phases correspond to that of a p-orbital on the central N atom. This accounts for the two different electron cloud phases as shown in the Gaussian surface.
• For MO 19, there is only one type of contributing ligand FO which is formed from the e' bonding fragment orbital. The overall MO is formed from having all ligand FOs form bonding interactions with the central N p-orbital. This produces the 4 layer phase as shown in the Gaussian surface. There is a strong through-bond interaction between the ligand FOs and the N p-orbital. Interestingly, there is no interaction between the ligand FOs. This is probably due to the fact that the through-space bonding interaction is so weak (due to their large separation from each other) that it does not provide significant stabilisation, and hence does not appear in the Gaussian surface.
• For MO 23, there is only one type of contributing ligand FO which is formed from the a1' antibonding fragment orbital. There is a strong through-bond antibonding interaction between the ligands and central N s-orbital; there is also a weak through-space bonding interaction between like-phases of the ligand FOs. These interactions result in the 3 layer phase as shown in the Gaussian surface. There is a red surface (representing the N s-orbital) that is difficult to see in the screenshot, however if done on Gaussian it does produce the same MO 23 which is antibonding.
Great description of the FO assignments with clear use of the BH3 fragments and good LCAOs. The MOs chosen show a good range of complexity, however, you were instructed to select only occupied MOs which MO23 isn’t and the assignment is a good suggestion but not quite correct.* Smf115 (talk) 22:21, 13 May 2019 (BST)
Overall a good report! The analysis section of your project is good and only let down by the lack of missing structure information and files for the ILs! Smf115 (talk) 22:21, 13 May 2019 (BST)
References
- ↑ J. Emsley, The Elements, 3rd edition, Clarendon Press, Oxford, 1998