Inorganic EBR16
BH3
B3LYP/3-21G level
Optimisation
Item Value Threshold Converged? Maximum Force 0.000217 0.000450 YES RMS Force 0.000105 0.000300 YES Maximum Displacement 0.000900 0.001800 YES RMS Displacement 0.000441 0.001200 YES
Smf115 (talk) 17:19, 28 May 2018 (BST)Nice to see the 3-21G calculation mentioned. The next step would be to mention why the basis set was improved to 6-31G(d,p) (this is additional information though!)
B3LYP/6-31G (d,p) level
Optimisation
Item Value Threshold Converged? Maximum Force 0.000049 0.000450 YES RMS Force 0.000032 0.000300 YES Maximum Displacement 0.000196 0.001800 YES RMS Displacement 0.000128 0.001200 YES
Frequency Analysis
Frequency analysis log file EBR_BH3_FREQ.log
Low frequencies --- -0.4059 -0.1955 -0.0054 25.3481 27.3325 27.3356 Low frequencies --- 1163.1913 1213.3139 1213.3166
Optimised Borane molecule |
IR - Molecular Vibrations
| Wavelength | Intensity | Symmetry | IR Active? | Type |
|---|---|---|---|---|
| 1163 | 93 | A2'' | YES | OUT-OF-PLANE BEND |
| 1213 | 14 | E' | YES | BEND |
| 1213 | 14 | E' | YES | BEND |
| 2582 | 0 | A1' | NO | SYMMETRIC STRETCH |
| 2715 | 126 | E' | YES | ASYMMETRIC STRETCH |
| 2715 | 126 | E' | YES | ASYMMETRIC STRETCH |
IR Spectrum
While there is six vibrational modes for BH3, there are two degenerate pairs and one IR inactive mode. The mode at 2582 cm-1 is the IR inactive and this is due to no net change in dipole moment. Then, the modes at 1213 cm-1 and 2715 cm-1 are the two degenerate pairs of modes so they overlap and are only seen as one peak per pair. Thus, the IR spectrum only shows three peaks present.
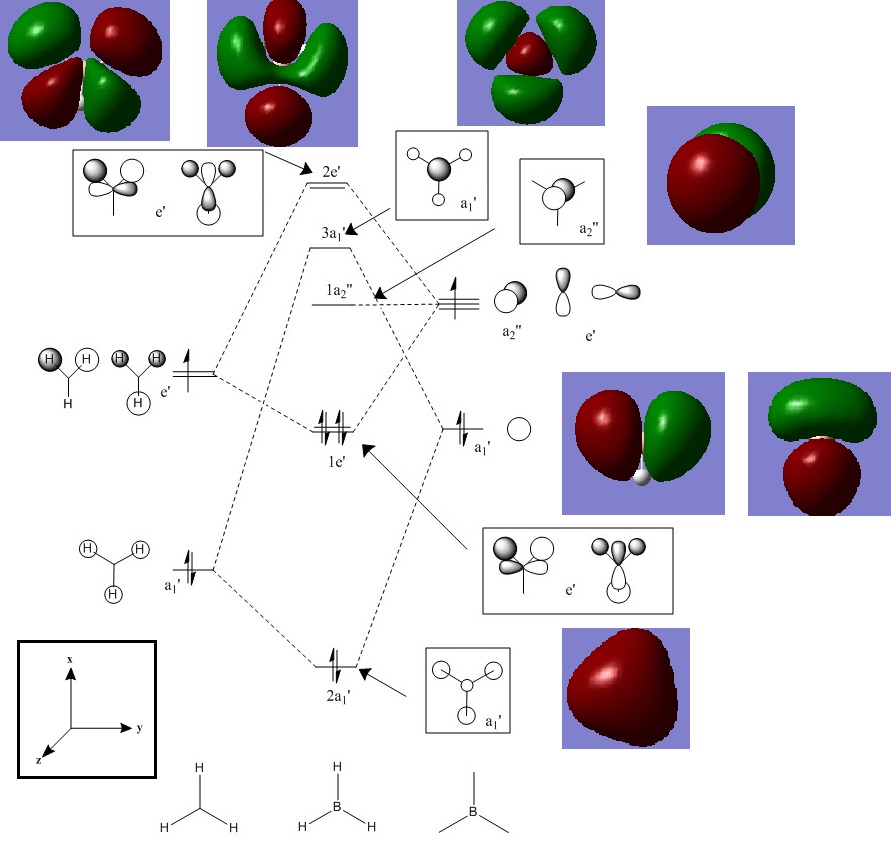
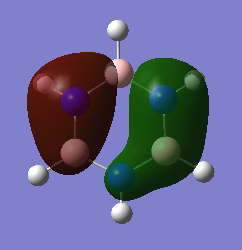
BH3 MO Diagram
Smf115 (talk) 17:20, 28 May 2018 (BST)Clear inclusion of the MOs in the diagram however, evaluation of the similarities and differences between the calculated and qualitative MOs is missing.
NH3
B3LYP/6-31G (d,p) level
Optimisation
Item Value Threshold Converged? Maximum Force 0.000006 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000012 0.001800 YES RMS Displacement 0.000008 0.001200 YES
Frequency Analysis
Frequency analysis log file EBR_NH3_FREQ.log
Low frequencies --- -11.6527 -11.6490 -0.0047 0.0332 0.1312 25.5724 Low frequencies --- 1089.6616 1694.1736 1694.1736
Optimised Ammonia molecule |
NH3BH3
B3LYP/6-31G (d,p) level
Optimisation
Item Value Threshold Converged? Maximum Force 0.000111 0.000450 YES RMS Force 0.000060 0.000300 YES Maximum Displacement 0.000662 0.001800 YES RMS Displacement 0.000436 0.001200 YES
Frequency Analysis
Frequency analysis log file EBR_NH3BH3_FREQ.log
Low frequencies --- 0.0006 0.0008 0.0014 16.6458 26.2463 40.6987 Low frequencies --- 266.5672 632.3100 639.3297
Optimised Aminoborane molecule |
Energy Calculations
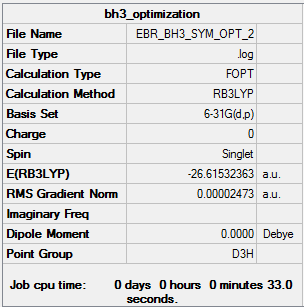
- E(BH3) = -26.61532363 au
- E(NH3) = -56.55776873 au
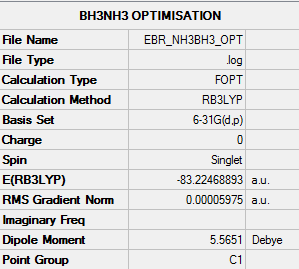
- E(NH3BH3) = -83.22468893 au
ΔE = E(NH3BH3) - [E(NH3)+E(BH3)] = -0.05159657 au
1 au = 1 hartree = 2625.50 kJmol-1
ΔE = -0.05159657 * 2625.50 = -135 kJmol-1
In order to evaluate how strong the B-N bond is, we can compare it to a C-C bond. Ethane is isoelectronic to aminoborane thus comparing the B-N and C-C bond (346 kJmol-1[1]) it can be inferred that the B-N is a weak bond as it is weaker than that of the analogous C-C species.
Smf115 (talk) 17:20, 28 May 2018 (BST)Correct calculation and a relevant comparison chosen to evaluate the strength of the bond.
BBr3
B3LYP/6-31G (d,p) level for B & LanL2DZ level for Br
Optimisation
Item Value Threshold Converged? Maximum Force 0.000008 0.000450 YES RMS Force 0.000005 0.000300 YES Maximum Displacement 0.000036 0.001800 YES RMS Displacement 0.000023 0.001200 YES
Frequency Analysis
Frequency analysis log file EBR_BBR3_FREQ.log
Low frequencies --- -0.0137 -0.0064 -0.0047 2.4315 2.4315 4.8421 Low frequencies --- 155.9631 155.9651 267.7052
Optimised BBr3 molecule |
Aromaticty
Benzene
B3LYP/6-31G (d,p) level
Optimisation
Item Value Threshold Converged? Maximum Force 0.000198 0.000450 YES RMS Force 0.000082 0.000300 YES Maximum Displacement 0.000849 0.001800 YES RMS Displacement 0.000305 0.001200 YES
Frequency Analysis
Frequency analysis log file EBR_BENZENE_FREQ.log
Low frequencies --- -11.6728 -0.0005 0.0005 0.0008 6.6686 15.6846 Low frequencies --- 414.0392 414.6031 621.0860
optimised Benzene molecule |
Borazine
B3LYP/6-31G (d,p) level
Optimisation
Item Value Threshold Converged? Maximum Force 0.000084 0.000450 YES RMS Force 0.000033 0.000300 YES Maximum Displacement 0.000276 0.001800 YES RMS Displacement 0.000077 0.001200 YES
Frequency Analysis
Frequency analysis log file EBR_BORAZINE_FREQ.log
Low frequencies --- -8.7403 -0.0006 -0.0005 -0.0005 9.0273 12.3082 Low frequencies --- 288.5748 290.5192 404.2343
Optimised Borazine molecule |
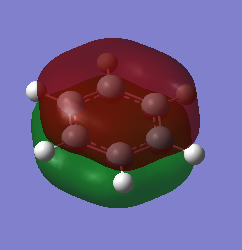
Charge Distribution Comparison
| Benzene | Borazine |
|---|---|
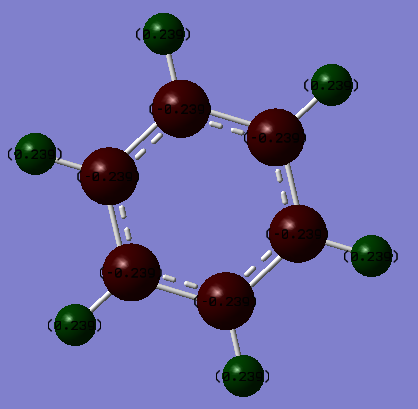
|
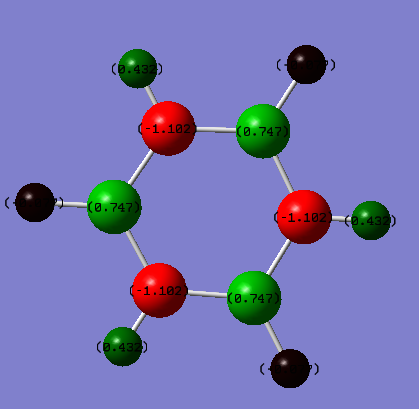
|
| Hydrogen = 0.239 | Hydrogen = -0.747 (bound to B) & 0.432 (bound to N) |
| Carbon = -0.239 | Boron = 0.747 Nitrogen = -1.102 |
These different charge distributions can be explained by considering the individual electronegativities of each constituent atom in the two molecules. For benzene, carbon (2.50[3]) is more electronegative than hydrogen (2.1[3]) is which leads to a slight positive charge forming on the hydrogen atoms which counteracts the negative forming on the carbon atoms. For borazine, nitrogen (3.07[3]) is more electronegative than hydrogen however boron (2.01[3]) is not; this leads to a negative charge forming on the hydrogens bound to boron, however a positive charge on the hydrogens bound to the nitrogen atoms. The electronegativity difference of the pairs (B&H vs. N&H) impacts the distribution of the charge; a smaller electronegativity difference between boron and hydrogen leads to a more even distribution of the charge (-0.747 to +0.747) whereas the higher electronegativity of nitrogen means the distribution is a lot more uneven (-1.102 to 0.432).
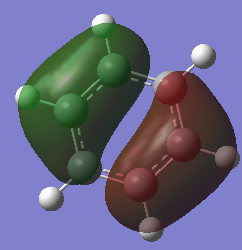
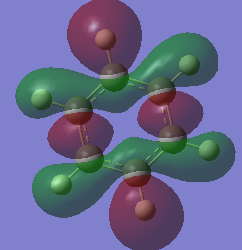
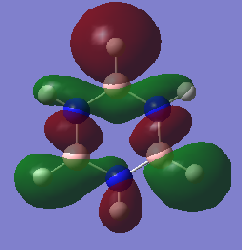
Molecular Orbital Comparisons
Smf115 (talk) 15:33, 1 June 2018 (BST)Nice selection of MOs chosen and attempt at comparing the MOs. To improve, the overall character and type (pi- or sigma-) of each MO should be identified and the comparison should be developed more, considering the effect of electronegative on the contribution in borazine for example.
Aromaticity Discussion
Aromaticity is defined by a number of criteria that a molecule must meet in order to be considered as aromatic. The requirement includes 4n+2 electrons in a cyclic, conjugated system and consequences of aromaticity include a great stabilisation compared to unsaturated analogues. The presence of aromaticity can be indicated by the bond lengths being intermediate of that of a single and double bond and the presence of a ring current when the molecule is in the presence of an external magnetic spin - due to the electron delocalisation.
Both benzene and borazine fulfill the criteria in order to be considered to be aromatic. Benzene consists of 6 carbon atoms, each contributing one p-electron to form a cyclic delocalised pi system above and below the plane of the ring. Borazine has an alternating ring of N and B atoms; the nitrogen atoms contributing one p-electron each and the borons have the presence of an empty orbital.
The real MO's indicate the delocalisation of electrons and show the overlapping of p orbitals to form the aromatic delocalised pi system. However, the concept of overlapping p orbitals alone is not sufficient enough to consider every situation in which aromaticity may arise. There is also the presence of sigma MO's in compounds which may help give rise to aromaticity of a molecule. It has recently been considered that, a molecule does not necessarily need to be planar in order for it to be considered aromatic. This has as such led to concepts such as quasi- and pseudo- aromaticity being developed in order to describe compounds with high aromatic stability but not quite displaying all other characteristics of a conventially aromatic molecule[4].
Smf115 (talk) 15:34, 1 June 2018 (BST)Overall, a decent wiki report which required more development and discussion in the latter half of the project section but had a very good first section.