Inorg 01188093
Part 1: Revision and Introduction
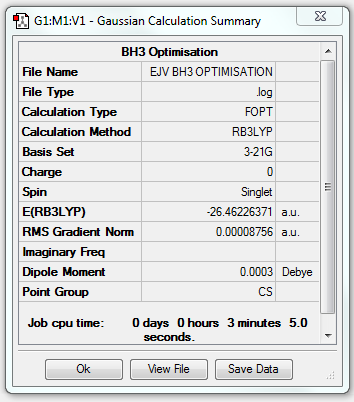
BH3
Basis Set: B3LYP/3-21G level
Item Value Threshold Converged? Maximum Force 0.000217 0.000450 YES RMS Force 0.000105 0.000300 YES Maximum Displacement 0.000919 0.001800 YES RMS Displacement 0.000441 0.001200 YES
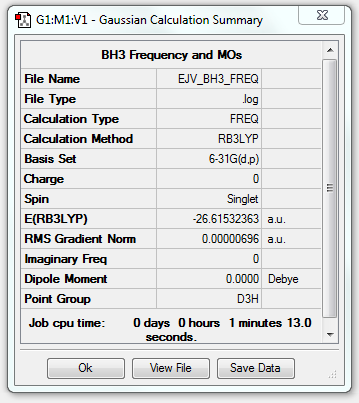
BH3
Optimisation
B3LYP/6-31G(d.p) level
Item Value Threshold Converged? Maximum Force 0.000014 0.000450 YES RMS Force 0.000007 0.000300 YES Maximum Displacement 0.000055 0.001800 YES RMS Displacement 0.000027 0.001200 YES
Frequency Analysis
Frequency analysis log file EJV_BH3_frequency.log
Low frequencies --- -7.9073 -1.6385 -0.0054 0.6256 6.5697 6.7709 Low frequencies --- 1162.9662 1213.1623 1213.1650
Jmol Dynamic Image
Optimised Borane |
Molecular Vibrations and Spectroscopy
WAVENUMBER (CM-1). INTENSITY(ARBITRARY UNITS). SYMMETRY. IR ACTIVE? TYPE 1163 93 A2" YES out-of-plane bend 1213 14 E' YES bend 1213 14 E' YES bend 2582 0 A1' NO symmetric stretch 2716 126 E' YES asymmetric stretch 2716 126 E' YES asymmetric stretch
In the table, there are six different vibrational modes but the IR spectrum only three peaks are seen. This is because there is one vibrational mode that is IR silent and two pairs of degenerate vibrations. As such, there are only three peaks in the IR spectrum.
The 4th vibration is not IR active as there is no change in dipole moment hence this vibrational mode does not appear in the IR spectrum. The 2nd and 3rd vibrational mode are degenerate as they both have the same wavenumber at 1213 cm-1. Similarly, the 5th and 6th vibrational modes are degenarate and as they have the same wavenumber at 2716 cm-1. In each of these cases only one peak will be observed instead of two as the vibrational modes have the same energy.
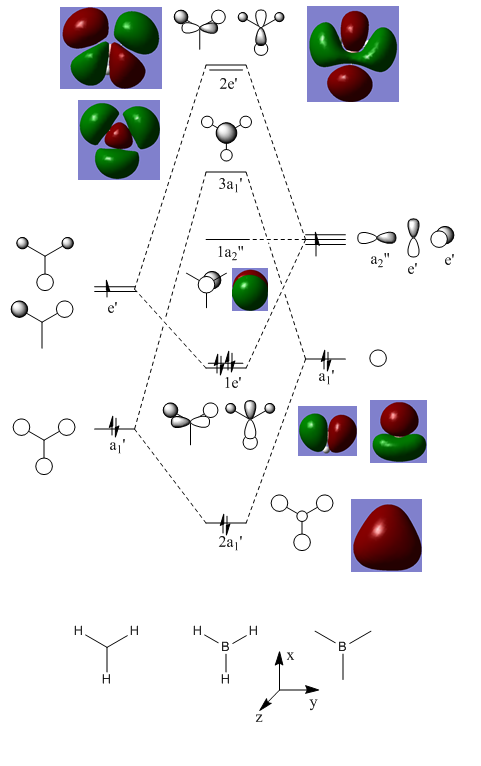
MO Diagram
Ng611 (talk) 13:49, 6 June 2018 (BST) From comparison of the qualitative MO diagram with the computed orbitals, what does this tell you about the validity of qualitative MO theory.
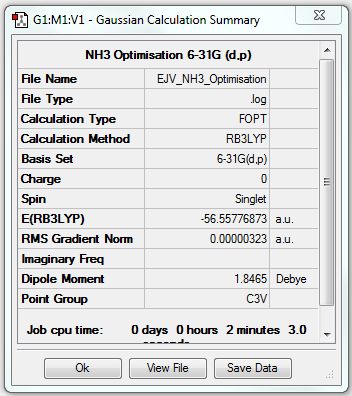
NH3
Optimisation
B3LYP/6-31G(d.p) level
Item Value Threshold Converged? Maximum Force 0.000006 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000012 0.001800 YES RMS Displacement 0.000008 0.001200 YES
Frequency Analysis
Frequency analysis log file: EJV_NH3_frequency.
Low frequencies --- -8.5646 -8.5588 -0.0044 0.0454 0.1784 26.4183 Low frequencies --- 1089.7603 1694.1865 1694.1865
Note: One of the low frequencies is 26 which is higher that 15 which is the value normally considered acceptable. Upon discussion with Dr. Hunt, this has been explained as the basis set not being accurate enough for this molecule and thus is acceptable for this value to be higher than normal.
Jmol Dynamic Image
Optimised Ammonia |
NH3BH3
Optimisation
B3LYP/6-31G(d.p) level
Item Value Threshold Converged? Maximum Force 0.000114 0.000450 YES RMS Force 0.000060 0.000300 YES Maximum Displacement 0.000722 0.001800 YES RMS Displacement 0.000356 0.001200 YES
Frequency Analysis
Frequency analysis log file EJV_NH3BH3_frequency.
Low frequencies --- -0.0011 -0.0010 0.0004 18.9364 22.2713 40.0725 Low frequencies --- 266.6488 632.2537 639.3716
Jmol Dynamic Image
Optimised NH3BH3 |
Energy Calculations
- E(BH3) = -26.61532363 au
- E(NH3) = -56.55776873 au
- E(NH3BH3) = -83.22468984 au
ΔE = E(NH3BH3) - [E(BH3) + E(NH3)]
∴ ΔE = -0.05159748 au
1 au = 2625.5 kJ mol-1
∴ ΔE = -135 kJ mol-1
Ng611 (talk) 13:50, 6 June 2018 (BST) Correct calcualtion and good consideration given to the accuracy of the reported result.
One way to examine the strength of the B-N bond is to compare it to the isoelectric species ethane. The strength of the C-C bond is -377.4 kJ mol-1 in ethane [1]. This shows that the B-N bond is weaker than the C-C bond in the analogous species.
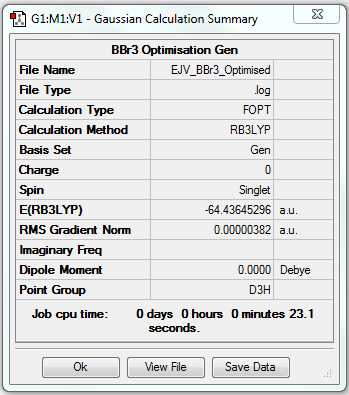
BBr3
Optimisation
B3LYP/6-31G(d.p) level for B and LanL2DZ level for Br
Item Value Threshold Converged? Maximum Force 0.000008 0.000450 YES RMS Force 0.000005 0.000300 YES Maximum Displacement 0.000036 0.001800 YES RMS Displacement 0.000023 0.001200 YES
Frequency Analysis
DSpace Link to Frequency Analysis log file: DOI:10042/202446 Frequency analysis log file EJV_BBr3_frequency.
Low frequencies --- -0.0137 -0.0064 -0.0046 2.4315 2.4315 4.8421 Low frequencies --- 155.9631 155.9651 267.7052
Jmol Dynamic Image
Optimised BBr3 |
Part 2: Aromaticity Project
Benzene
Optimisation
B3LYP/6-31G(d.p) level
Item Value Threshold Converged? Maximum Force 0.000198 0.000450 YES RMS Force 0.000082 0.000300 YES Maximum Displacement 0.000849 0.001800 YES RMS Displacement 0.000305 0.001200 YES
Frequency Analysis
Frequency analysis log file EJV_Benzene_Frequency.
Low frequencies --- -11.6728 0.0002 0.0005 0.0005 6.6686 15.6846 Low frequencies --- 414.0392 414.6031 621.0860
Optimised Benzene |
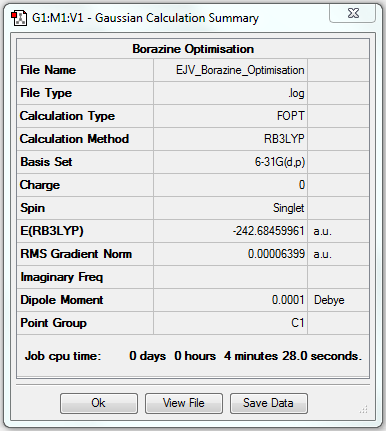
Borazine
Optimisation
B3LYP/6-31G(d.p) level
Item Value Threshold Converged? Maximum Force 0.000085 0.000450 YES RMS Force 0.000033 0.000300 YES Maximum Displacement 0.000249 0.001800 YES RMS Displacement 0.000077 0.001200 YES
Frequency Analysis
Frequency analysis log file EJV_Borazine_Frequency.
Low frequencies --- -17.7200 -11.7048 -9.5404 -0.0009 -0.0007 -0.0005 Low frequencies --- 288.9793 289.5055 404.2272
Optimised Borazine |
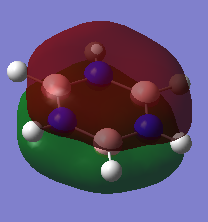
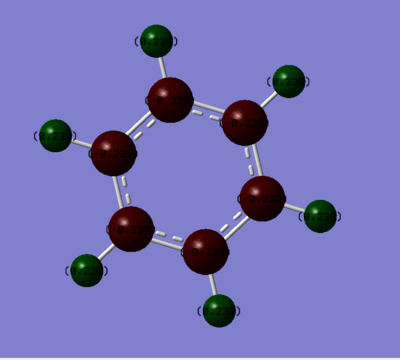
Charge Distribution Comparison
| Benzene | Borazine |
|---|---|
|
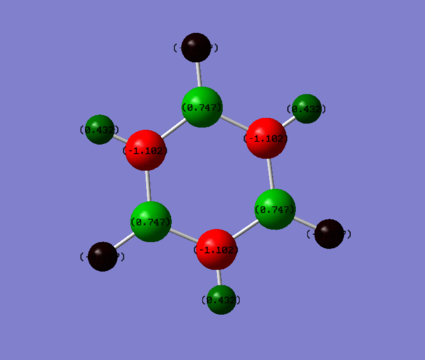
|
| Hydrogen: 0.239 | Hydrogen bonded to Boron: -0.747 , Hydrogen bonded to Nitrogen: 0.432 |
| Carbon: -0.239 | Boron: 0.747, Nitrogen: -1.102 |
We can explain the different charge densities by examining the electronegativity of the atoms involved in the molecule.
We would expect that there would be little polarization of the bonds withing benzene with the values remaining near to 0 as there is a small electronegativity difference between carbon and hydrogen. The values according to the Pauling scale is 2.50 [2] and 2.10 [3] for Carbon and Hydrogen respectively. This explains why there is a small charge distribution. As C is slightly more electronegative it withdraws the electron density in the bond slightly and results in a small negative charge on the C and a small positive charge on the H.
In Borazine, Nitrogen is more electronegative that H resulting in a a negative charge developing on the Nitrogen. The values according to the Pauling scale is 3.07 and 2.10 [2] for Nitrogen and Hydrogen respectively. The difference in charge between N an H is greater than in benzene as there is a greater gap in electronegativity between N and H compared to C and H. In contrast, Boron is more electropositive than Hydrogen and therefore the hydrogen withdraws the electrons in the covalent bond towards itself and this results in a negative charge building up on the hydrogen this time and a positive charge building up on the Boron.
Ng611 (talk) 13:51, 6 June 2018 (BST) Good discussion of the effects of electronegativity on the overall charge distribution. What do the partial charges sum to, and is there any difference in partial charge for atoms related by symmetry?
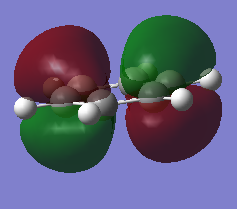
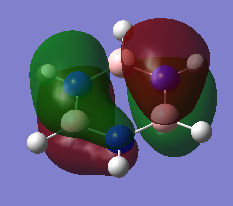
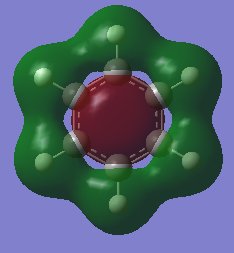
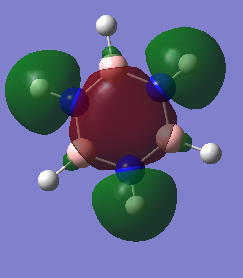
Molecular Orbitals Comparison
Ng611 (talk) 13:54, 6 June 2018 (BST) Some good analysis, although a little inconsistent. For example, for the 2nd and 3rd orbitals, you discuss differences in electronegativity, but not for the first orbital. How also is the symmetry and point group of the molecules reflected in the orbitals? Remember to also give the directions of orbitals where possible (i.e.: in plane/ out of plane 2p, etc).
Description of Aromaticity
First used to explain the relative stability of benzene in comparison to its olefenic counterpart the concept of aromaticity was introduced. This stability is attributed to the cyclic delocalisation of the pi electrons in benzene and other related molecules. The delocalised pi system results in four ground state properties:
- The first has already been mentioned, which is the increase in stability of benzene in comparison to the theoretical cyclohexatriene.
- The second is the bond lengths are the intermediate between the double bond and the single bond
- Due to the ring current, this increases the NMR chemical shift of hydrogens outside of the ring
- Aromatic systems undergo aromatic substitution and thus preserve the aromatic system.[4]
It was also established that in order for the system to be aromatic there must be 4n+2 pi electrons in contiguous p-orbitals.
Both benzene and borazine are consistent with these rules. In benzene, on each C there is a p orbital which doesn't lay in the plane of the molecule which contains one electron each. There are six carbons, each donating one electron to the system thus resulting in 6 electrons in the delocalised pi system. This therefore fits with the Huckles rule. Borazine on the other hand, the nitrogen atoms donate 2 electrons each from their p(z) into the empty p(z) orbitals on the boron. Again, this results in 6 electrons being in the delocalised pi system again, fitting with the Huckles Rule. We can see this classical picture of aromaticity above in the MOs of benzene and borazine.
Recent advancements in chemistry contradict the classical basis for aromaticity. Quasi aromatic systems are systems are systems that do not look like benzene but exhibit some but not all of the criteria listed above. Pseudo-aromatic systems are systems that look like benzene but do not display any of the properties mentioned above. This has lead to criticism of the basic model of aromaticity (overlapping of contiguous planar p oritals) and the suggestion that there is a quatum mechanical explanation of aromaticity.
Ng611 (talk) 13:58, 6 June 2018 (BST) A good basic introduction to aromaticity in the first two paragraphs. In your final paragraph, you go into some detail regarding more modern concepts of aromaticity, which are interesting. Expanding on these points would have strengthened this discussion significantly.
Ng611 (talk) 13:58, 6 June 2018 (BST) Overall, a good report. Accurate calculations and a really strong first section, but you let yourself down in the second section by not including enough detail in your answers.
References
- ↑ Y. R. Luo, "Comprehensive Handbook of Chemical Bond Energies", CRC Press, Boca Raton, Florida, 2007
- ↑ 2.0 2.1 E. J. Little, M. M. Jones, J. Chem. Educ., 1960, 37, 231-233.
- ↑ L. Pauling, “The Nature of the Chemical Bond,” 2nd ed., Cornell University Press, Ithaca, New York, 1942
- ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedAromaticity