InorgCompafg216
Day 1
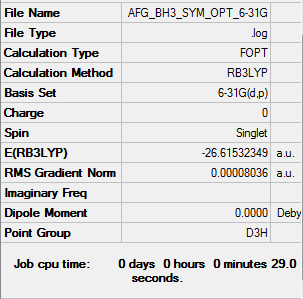
BH3 Optimisation: RB3LYP/6-31G(d,p) level
Item Value Threshold Converged? Maximum Force 0.000161 0.000450 YES RMS Force 0.000105 0.000300 YES Maximum Displacement 0.000638 0.001800 YES RMS Displacement 0.000417 0.001200 YES
Low frequencies --- -0.2456 -0.1129 -0.0054 44.0270 45.1846 45.1853 Low frequencies --- 1163.6049 1213.5924 1213.5951
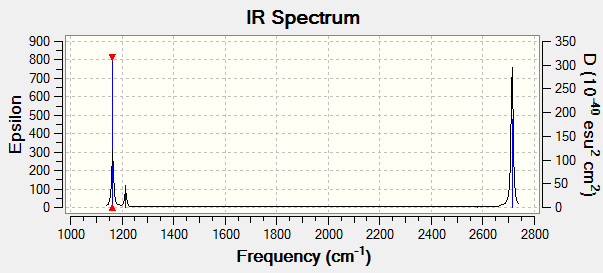
Vibrational spectrum for BH3
| wavenumber (cm-1 | Intensity (arbitrary units) | symmetry | IR active? | type |
| 1164 | 92 | A2 | yes | out-of-plane bend |
| 1214 | 14 | E' | yes | in-plane bend |
| 1214 | 14 | E' | yes | in-plane bend |
| 2580 | 0 | A1' | no | symmetric stretch |
| 2713 | 126 | E' | yes | asymmetric stretch |
| 2713 | 126 | E' | yes | asymmetric stretch |
We have fewer than 6 peaks in the spectrum despite observing 6 peaks computationally due to the symmetry of the molecule. The second and third vibrations and the fifth and sixth vibrations are degenerate, and the fourth vibration causes no change in dipole moment due to symmetry and thus will not appear.
Smf115 (talk) 01:04, 23 May 2018 (BST)Correctly assigned symmetries and vibrational modes. The comment highlighted both the degenerate modes and the IR inactive vibration which was good.
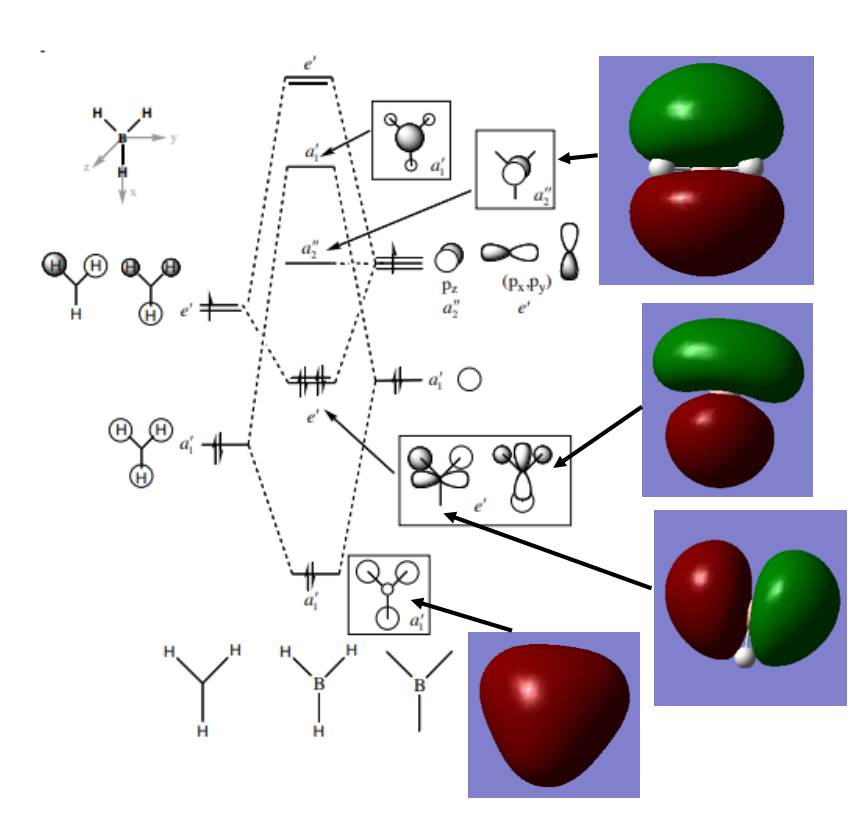
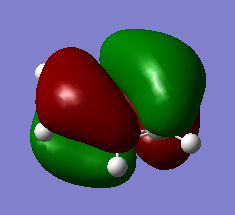
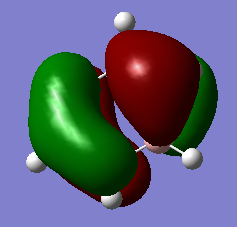
If the LCAO representations of occcupied orbitals are combined visually by phase, there are no obvious differences between the real and LCAO MOs. This reflects well on qualitative MO theory, suggesting that it is indeed a very accurate representation of reality. More differences arise when we consider unoccupied MOs however.
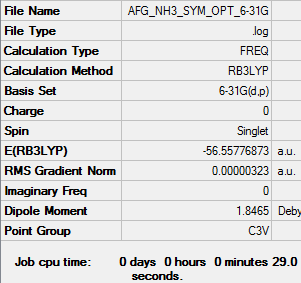
NH3 Optimisation: RB3LYP/6-31G(d,p) level
Item Value Threshold Converged? Maximum Force 0.000006 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000014 0.001800 YES RMS Displacement 0.000009 0.001200 YES
Media:AFG_NH3_SYM_OPT_6-31G.LOG
Low frequencies --- -0.0127 -0.0001 0.0010 7.1034 8.1048 8.1051 Low frequencies --- 1089.3834 1693.9368 1693.9368
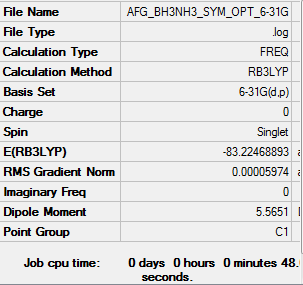
NH3BH3 Optimisation: RB3LYP/6-31G(d,p) level
Item Value Threshold Converged? Maximum Force 0.000123 0.000450 YES RMS Force 0.000058 0.000300 YES Maximum Displacement 0.000585 0.001800 YES RMS Displacement 0.000320 0.001200 YES
Media:AFG_BH3NH3_SYM_OPT_6-31G.LOG
Low frequencies --- -0.0020 -0.0011 -0.0011 16.8436 17.4462 37.3291 Low frequencies --- 265.8243 632.2043 639.3227
Optimised NH3BH3 Jmol Animation
The B-N Bond in NH3BH3
E(NH3) = -56.558 a.u.
E(BH3) = -26.614 a.u.
E(NH3BH3) = -83.224 a.u.
ΔE = E(NH3BH3) - [E(NH3) + E(BH3)]
ΔE = -83.224 - (-56.558 + -26.614)
ΔE = -0.052 a.u. or -140 kJ/mol
We can conclude that this bond energy of the B-N dative bond in NH3BH3 is relatively weak when we compare it to other known common bond energies, like H-H at 432 kJ/mol and C-C at 411 kJ/mol. Even when we compare it to bonds regarded as weak it comes out the weaker; C-I at 213 kJ/mol and H-I at 295 kJ/mol are both stronger.
Smf115 (talk) 01:02, 23 May 2018 (BST)Correct calculation method and consideration towards accuracy. However, the numbers being manipulated have been rounded too early resulting in an incorrect final answer. The comparison used within the comment is good but literature values must be referenced.
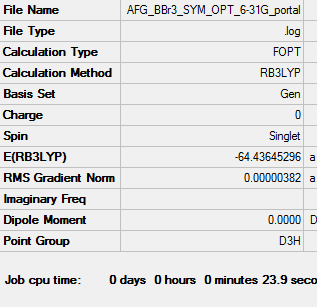
BBr3 Optimisation: RB3LYP/Gen level
Item Value Threshold Converged? Maximum Force 0.000008 0.000450 YES RMS Force 0.000005 0.000300 YES Maximum Displacement 0.000036 0.001800 YES RMS Displacement 0.000023 0.001200 YES
Frequency analysis file: DOI:10042/202387
Low frequencies --- -0.0137 -0.0064 -0.0046 2.4315 2.4315 4.8421 Low frequencies --- 155.9631 155.9651 267.7052
Days 2-3: Aromaticity Mini Project
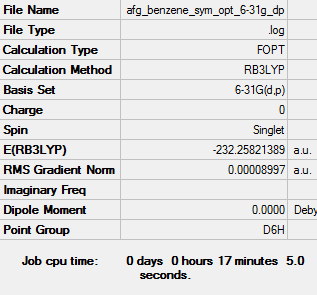
Benzene Optimisation: RB3LYP/6-31G(d,p) Level
Item Value Threshold Converged? Maximum Force 0.000202 0.000450 YES RMS Force 0.000078 0.000300 YES Maximum Displacement 0.000823 0.001800 YES RMS Displacement 0.000287 0.001200 YES
Frequency analysis file: Media:AFG_BENZENE_FREQ.LOG
Low frequencies --- -2.5530 -2.5530 -0.0088 -0.0042 -0.0042 10.3930 Low frequencies --- 413.9723 413.9723 621.1358
Optimised Benzene Jmol Animation
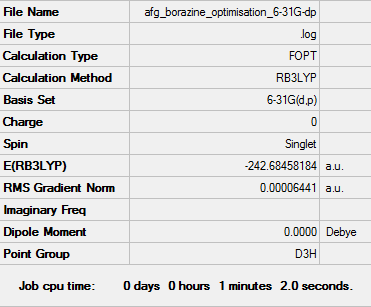
Borazine Optimisation: RB3LYP/6-31G(d,p) Level
Item Value Threshold Converged? Maximum Force 0.000086 0.000450 YES RMS Force 0.000033 0.000300 YES Maximum Displacement 0.000253 0.001800 YES RMS Displacement 0.000075 0.001200 YES
Frequency analysis file: Media:AFG_BENZENE_FREQ.LOG
Low frequencies --- -12.7064 -12.4838 -9.0645 -0.0097 0.0412 0.0663 Low frequencies --- 289.1165 289.1269 403.9186
Optimised Borazine Jmol Animation
Smf115 (talk) 00:16, 23 May 2018 (BST)Good structure information for both molecules, however, the borazine log file also links to the benzene one.
Charge Distribution Comparison
| Molecule | Charge Distribution | Coloured Charge Distribution |
| Benzene | 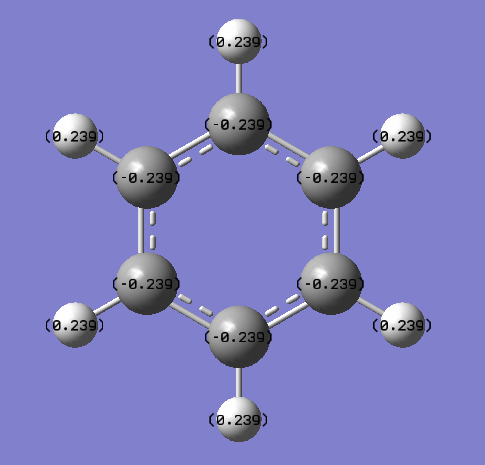
|
|
| Borazine | 
|
|
| Benzene | |||
| C Partial Charge | H Partial Charge | ||
| -0.239 | 0.239 | ||
| Borazine | |||
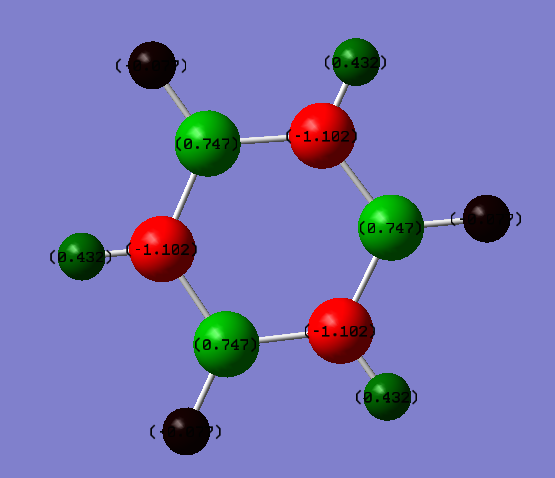
| N Partial Charge | B Partial Charge | H (N) Partial Charge | H (B) Partial Charge |
| -1.102 | 0.747 | 0.432 | -0.077 |
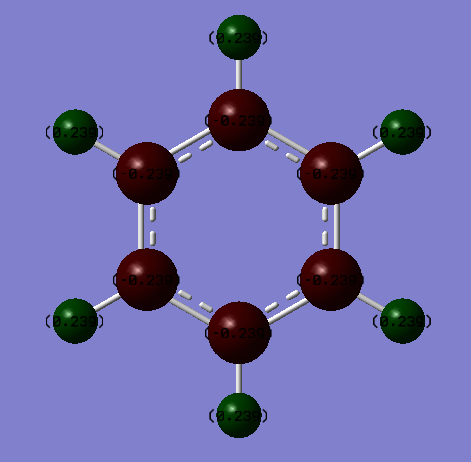
Charge is symmetrically distributed in benzene, with every atom in the ring having a partial negative charge of -0.239 and every hydrogen a partial charge of 0.239. This reflects the relatively non-polar and stable nature of the C-H bond. Also the slightly greater electronegativity of carbon (2.5 on the Pauling scale) than of hydrogen (2.1 on the Pauling scale) is reflected by the C atoms having a negative charge and the H atoms the positive charge; electronegativity is defined as the ability of an atom to attract electrons (and thus negative charge) towards itself in a covalent bond. The charge is evenly spread around the circumference of the ring as all carbon atoms have the same electronegativity.
There is a much greater separation in the distribution of charge in borazine due to the greater difference in electronegativity between boron, nitrogen and hydrogen (2.0, 3.0 and 2.1 on the Pauling scale respectively). Consequently nitrogen has a much larger amount of negative partial charge than both boron and the two types of hydrogen. The H atoms attached to N atoms have a significant positive partial charge as they are significantly less electronegative (1.0 on the Pauling scale) than the N atoms, and the H atoms attached to B atoms have a smaller but negative charge by the same reasoning - their electronegativity is larger than that of boron, but the difference is not as large so the charge separates less.
It should be noted that all charges given are in units of fundamental electron charge and all charges in both molecules sum to zero as they each have overall neutral charge.
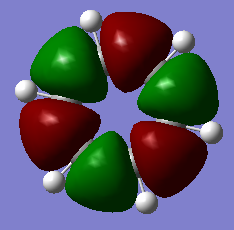
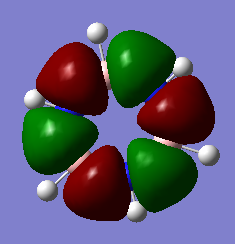
MO Visualisation and Discussion
Smf115 (talk) 01:00, 23 May 2018 (BST)Nice discussion of the MOs but it would have been nicer to see a wider range of bonding/anti-bonding character chosen. The top MO is perhaps more bonding due to the nodes positioned from the original p-orbitals and strong sigma-type interactions between the in-phase lobes on adjacent atoms around the ring.
Aromaticity Discussion
The IUPAC Gold Book defines aromaticity as 'the concept of spatial and electronic structure of cyclic molecular systems displaying the effects of cyclic electron delocalization which provide for their enhanced thermodynamic stability (relative to acyclic structural analogues) and tendency to retain the structural type in the course of chemical transformations'1. Practically, the aromaticity of a molecule can be ascertained from the shape of a molecule and from the nature of the orbitals present; Huckel's define a molecule to be aromatic if:
1) it is planar
2) it is cyclic (containing a continuous ring of atoms)
3) it is fully conjugated (the p orbitals of each atom line up in phase)
4) it contains 4n+2 pi electrons (where n=0,1,2,3...).
Aromaticity is reflected, as stated in the Gold Book, by significantly increased stability when compounds are aromatic, become aromatic or pass through an aromatic intermediate stage when reacting.
However, at 20 K, it has been observed that crystalline benzene adopts a non-planar chair conformation while retaining its aromaticity; this is one example of many cases where the planarity of aromatic molecules can be disrupted quite easily2. This leads us to believe that the model suggesting that aromaticity is strongly linked to overlap of adjacent pz atomic orbitals might be misinformative. It has been suggested that sigma orbitals might also contribute significantly to aromaticity of molecules.
More recently it has thus been suggested that aromaticity can be defined by a few properties2:
1) aromatic structures have an additional stability when compared to their olefin equivalents
2) they have intermediate bond lengths (between the lengths of standard single and double bonds)
3) an external magnetic field can induce a ring current (through the conjugated cyclic pi orbitals), the effect of which is observable in 1H NMR.
The 'real' MOs we have generated here can go some way to explain the delocalisation stability observed in aromatic molecules. Above, in the orbitals at levels 17 and 21 for both borazine and benzene, we can see orbitals which spread across multiple atoms, over the top and bottom of the planes. These orbitals suggest that the pi electrons within them are allowed to delocalise across the plane, which is a key idea in qualitative descriptions of aromaticity.
1. IUPAC Gold Book; https://goldbook.iupac.org/html/A/A00442.html; accessed 11/5/18
2. Application of AIM Parameters at Ring Critical Points for Estimation of p-Electron Delocalization in Six-Membered Aromatic and Quasi-Aromatic Rings, M. Palusiak and T. M. Krygowski; https://onlinelibrary.wiley.com/doi/epdf/10.1002/chem.200700250; DOI: 10.1002/chem.200700250; Chem. Eur. J. 2007, 13, 7996 – 8006
Smf115 (talk) 00:56, 23 May 2018 (BST)Great answer discussiong both the basic and more complex ideas around aromaticity. Well referenced examples from relevant literature and good consideration towards the MOs just visualised.
Smf115 (talk) 00:56, 23 May 2018 (BST)Overall a good report with a strongly attempted project section.