InComLabYuhan
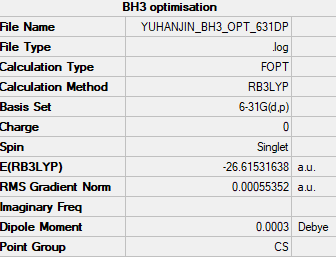
BH3
RB3LYP/6-31G(d,p) level
Summary Table
Item Value Threshold Converged? Maximum Force 0.000014 0.000450 YES RMS Force 0.000007 0.000300 YES Maximum Displacement 0.000055 0.001800 YES RMS Displacement 0.000027 0.001200 YES
Item Table
Frequency Table
Low frequencies --- -7.9073 -1.6385 -0.0055 0.6256 6.5697 6.7709 Low frequencies --- 1162.9662 1213.1623 1213.1650
Frequency analysis log file YUHANJIN_BH3_OPT_631DP_-_COPY_EDITED_D3H_FREQ.LOG
Borane |
The following four images show the calculated MOs for BH3.
Ng611 (talk) 00:56, 15 May 2019 (BST) Which calculated MOs correlate to which MOs on the orbital diagram?
The picture above was taken from Tutorial problems for Lecture 4, made by Prof. Patricia Hunt.
The size of atoms in real MOs is more realistic as three H atoms are much smaller than B as shown. In LCAO, size of atoms will change in order to indicate the contribution of particular atom to the MOs;
Also, the diffusion of electron density could be proven by real MOs since we could see a total red 'sphere' for 2a1' orbital.
The rest two orbitals are quite similar for both LCAO and real MOs.
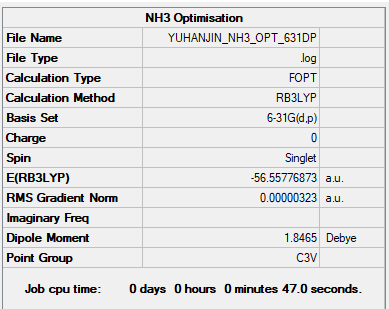
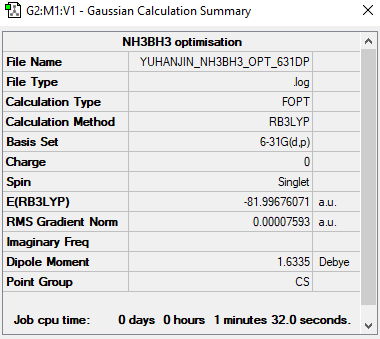
NH3 and NH3BH3
Ng611 (talk) 00:58, 15 May 2019 (BST) We also needed frequency log files, convergence tables, low frequencies, and jmol images for both of these molecules.
E(NH3)= -56.55777 a.u. E(BH3)= -26.61532 a.u. E(NH3BH3)= -81.99676 a.u.
Ng611 (talk) 00:58, 15 May 2019 (BST) BH3 and NH3 values are good, but the energy of your BH3NH3 adduct is off by quite a bit.
Association Energy ΔE=E(NH3BH3)-[E(NH3)+E(BH3)] = 1.17633 a.u. = 738kJ/mol Based on the calculation, the bond strength of B-N bond is strong. With comparison of N-N 167 kJ/mol and B-B 293 kJ/mol. (accessed at http://www.wiredchemist.com/chemistry/data/bond_energies_lengths.html) Therefore, the B-N bond has ionic character which strengthen the bond.
Ng611 (talk) 01:00, 15 May 2019 (BST) I'd choose different comparisons. An isoelectronic bond would have been more appropriate. Also, you should use book sources where possible.
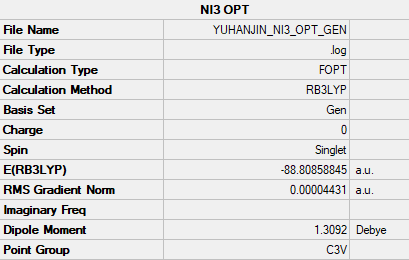
NI3
RB3LYP/Gen level
Summary Table
Item Value Threshold Converged? Maximum Force 0.000102 0.000450 YES RMS Force 0.000075 0.000300 YES Maximum Displacement 0.000667 0.001800 YES RMS Displacement 0.000490 0.001200 YES
Item Table
Low frequencies --- -12.3847 -12.3783 -5.6131 -0.0040 0.0194 0.0711 Low frequencies --- 100.9307 100.9314 147.2333
Frequency analysis log file YUHANJIN_NI3_OPT_GEN_FREQ3.LOG
NI3 |
The optimised N-I bond distance is 2.184 Å
Metal Carbonyls
Ng611 (talk) 01:13, 15 May 2019 (BST) Several parts of this section are missing. You needed to include overall charges in your calculations (where apprpriate, e.g.: your Ti and Fe complexes). You also needed to perform an LCAO decomposition on your complex MOs, and choose (and explain) a relevant trend.
RB3LYP/Gen
[Ti(CO)6]2-
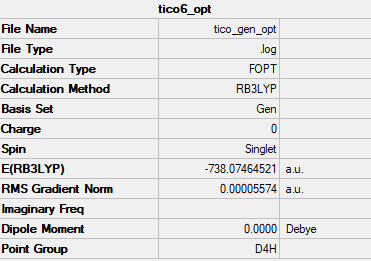
Summary table
Bond length (Ti-C) = 2.160 Å
Vibrational frequencies:
Low frequencies --- -3.6954 -0.0013 -0.0011 -0.0009 12.8157 12.8159 Low frequencies --- 15.4924 15.4925 29.1347
Frequency analysis log file TICO_GEN_OPT_FREQ.LOG
Ng611 (talk) 01:02, 15 May 2019 (BST) You're missing your conversion table here.
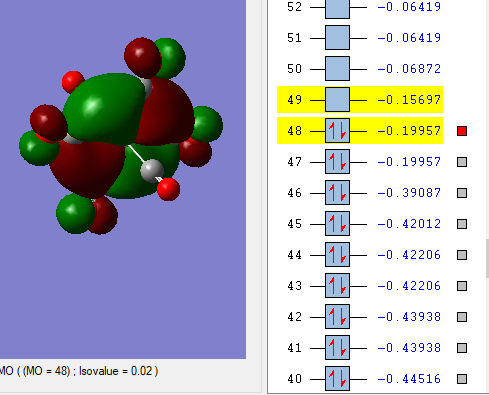
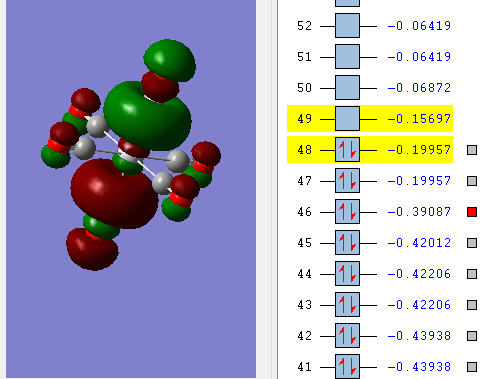
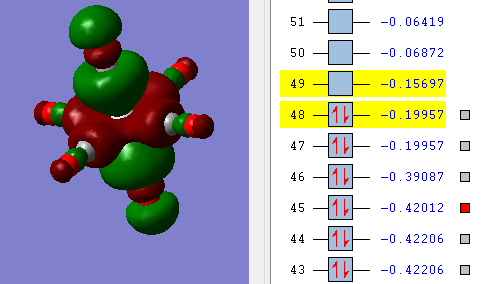
Three diagrams below showing three valence MOs calculated.
This MO is HOMO of [Ti(CO)6]2-.
The overall bonding is strong anti-bonding character.
There is in-ligand pi-antibonding on four ligands in the plane and ligand-ligand pi-antibonding.
Overall MO bonding is anti-bonding character. There is in-ligand pi-antibonding on four ligands in the plane. There is a 'donut-like' MO along the z-axis. The ligand-ligand interaction is anti-bonding. The ligands at equatorial positions have same types MOs.
Overall MO bonding is bonding character. The in-ligand bonding of four ligands is pi-antibonding. Along z-direction, in-ligand bonding is pi-antibonding.
[Ti(CO)6]2- |
Cr(CO)6
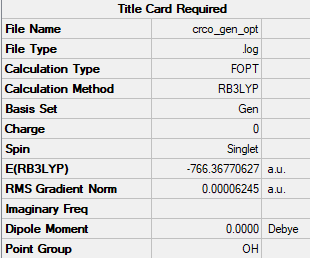
Summary table
bond length (Cr-C) = 1.915 Å
Item Value Threshold Converged? Maximum Force 0.000110 0.000450 YES RMS Force 0.000041 0.000300 YES Maximum Displacement 0.000709 0.001800 YES RMS Displacement 0.000336 0.001200 YES
vibrational frequencies:
Low frequencies --- -0.0006 -0.0003 0.0007 11.7424 11.7424 11.7424 Low frequencies --- 66.6546 66.6547 66.6547
Frequency analysis log file CRCO_GEN_OPT_FREQ.LOG
Cr(CO)6 |
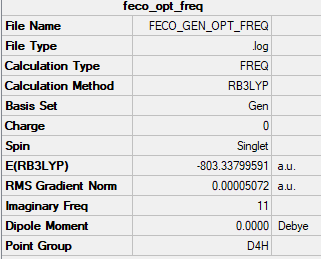
[Fe(CO)6]2+
Summary table
bond length (Fe-C) = 2.136 Å
Item Value Threshold Converged? Maximum Force 0.000175 0.000450 YES RMS Force 0.000039 0.000300 YES Maximum Displacement 0.000494 0.001800 YES RMS Displacement 0.000138 0.001200 YES
vibrational frequencies
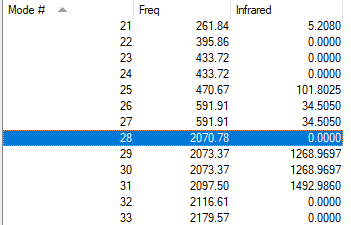
Low frequencies --- -572.6144 -572.6144 -387.4436 -340.7770 -323.3723 -289.6696 Low frequencies --- -289.6696 -249.5631 -105.2159
Frequency analysis log file FECO_GEN_OPT_FREQ.LOG
[Fe(CO)6]2+ |
Main group halides
RB3LYP/Gen
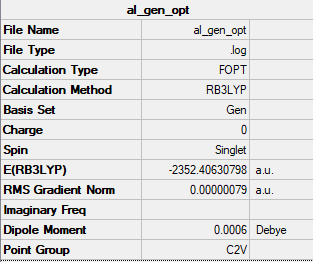
isomer (a)
relative energy = -2352.40631 a.u. = 6.17624277*10^6 kJ/mol
Item Value Threshold Converged? Maximum Force 0.000002 0.000450 YES RMS Force 0.000001 0.000300 YES Maximum Displacement 0.000642 0.001800 YES RMS Displacement 0.000255 0.001200 YES
Low frequencies --- -5.1634 -5.0501 -3.1849 0.0022 0.0028 0.0041 Low frequencies --- 14.8310 63.2729 86.0759
Frequency analysis log file AL_GEN_OPT_FREQ.LOG