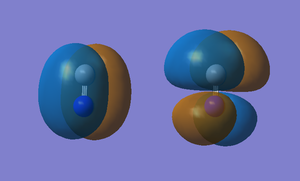IMM2 MS
Ammonia - NH3
Optimisation Data
| Optimisation Data | Value |
|---|---|
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d.p) |
| Final Energy (E(RB3LYP)) | -56.55776873 a.u. |
| RMS Gradient | 0.00000485 a.u. |
| Point Group | C3v |
| N-H Bond Length | 1.01798 Å |
| H-N-H Bond Angle | 105.741° |
Item Value Threshold Converged?
Maximum Force 0.000004 0.000450 YES
RMS Force 0.000004 0.000300 YES
Maximum Displacement 0.000072 0.001800 YES
RMS Displacement 0.000035 0.001200 YES
Predicted change in Energy=-5.986293D-10
Optimization completed.
-- Stationary point found.
----------------------------
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,2) 1.018 -DE/DX = 0.0 !
! R2 R(1,3) 1.018 -DE/DX = 0.0 !
! R3 R(1,4) 1.018 -DE/DX = 0.0 !
! A1 A(2,1,3) 105.7412 -DE/DX = 0.0 !
! A2 A(2,1,4) 105.7412 -DE/DX = 0.0 !
! A3 A(3,1,4) 105.7412 -DE/DX = 0.0 !
! D1 D(2,1,4,3) -111.8571 -DE/DX = 0.0 !
--------------------------------------------------------------------------------
GradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGrad
Ammonia Molecule |
The optimisation file is linked to here
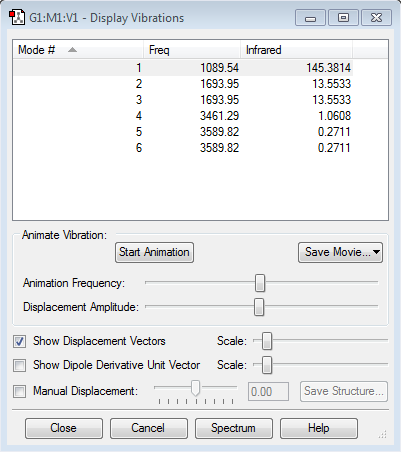
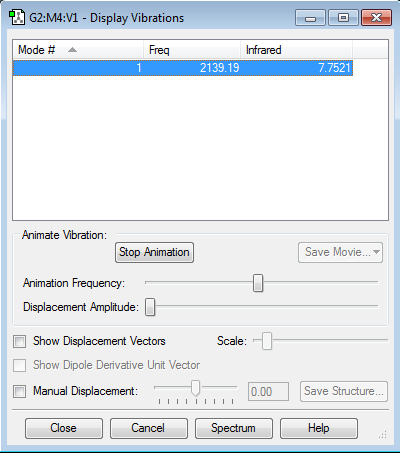
"Display Vibrations" Window
Questions
Expected number of modes => (3x4)-6 = 12-6 = 6 modes
Degenerate Modes => Modes 2 & 3 and 5 & 6 are degenerate frequency mode pairs
Mode Vibration => Modes 1,2 and 3 are 'bending' vibrations, Modes 4,5 and 6 are 'streching' vibrations
Highly Symmetric Mode => Mode 4 is highly symmetric
"Umbrella" Mode =>Mode 1 is the "Umbrella" mode
Expected Bands => The data shows that a spectrum will have 2 bands at 1089.54 cm-1 and 1693.95 cm-1, for mode 1 and modes 2 & 3 respectively. modes 4, 5 and 6 have no bands as these modes don't have a change in dipole moment and so are IR inactive.
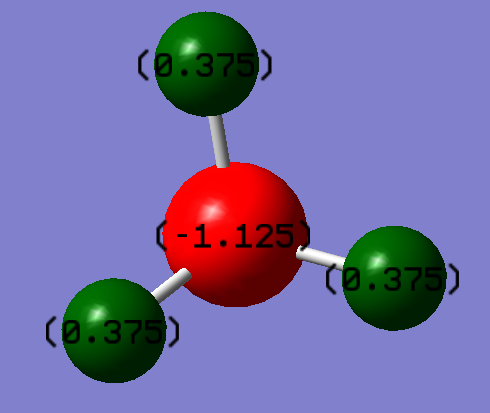
Charge Distribution
| Atom | Charge |
|---|---|
| N | -1.125 |
| H | 0.375 |
It would be expected for the Nitrogen atom to carry a negative charge as N is more electronegative than Hydrogen, which is also why a positive charge is expected on the H atoms.
Nitrogen - N2
Optimisation Data
| Optimisation Data | Value |
|---|---|
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d.p) |
| Final Energy (E(RB3LYP)) | -109.52412868 a.u. |
| RMS Gradient | 0.00000060 a.u. |
| Point Group | C∞h |
| N-N Bond Length | 1.10550 Å |
Item Value Threshold Converged?
Maximum Force 0.000001 0.000450 YES
RMS Force 0.000001 0.000300 YES
Maximum Displacement 0.000000 0.001800 YES
RMS Displacement 0.000000 0.001200 YES
Predicted change in Energy=-3.401033D-13
Optimization completed.
-- Stationary point found.
----------------------------
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,2) 1.1055 -DE/DX = 0.0 !
--------------------------------------------------------------------------------
GradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGrad
Nitrogen Molecule |
The optimisation file is linked to here
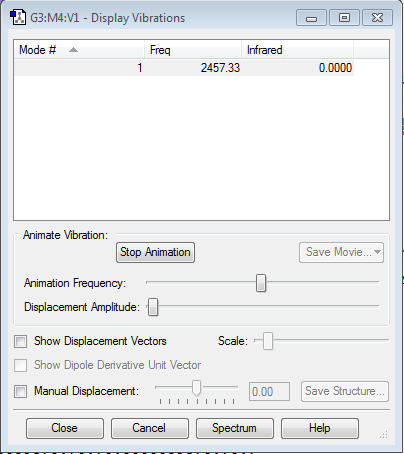
"Display Vibrations" Window
1 Mode of Vibration - no negative frequencies. The vibration is IR inactive as there is no change in dipole moment (molecule is homo-nuclear diatomic).
Hydrogen - H2
Optimisation Data
| Optimisation Data | Value |
|---|---|
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d.p) |
| Final Energy (E(RB3LYP)) | -1.17853935 a.u. |
| RMS Gradient | 0.00005349 a.u. |
| Point Group | C∞h |
| H-H Bond Length | 0.74292 Å |
Item Value Threshold Converged?
Maximum Force 0.000093 0.000450 YES
RMS Force 0.000093 0.000300 YES
Maximum Displacement 0.000122 0.001800 YES
RMS Displacement 0.000172 0.001200 YES
Predicted change in Energy=-1.129292D-08
Optimization completed.
-- Stationary point found.
----------------------------
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,2) 0.7429 -DE/DX = -0.0001 !
--------------------------------------------------------------------------------
GradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGrad
Hydrogen Molecule |
The optimisation file is linked to here
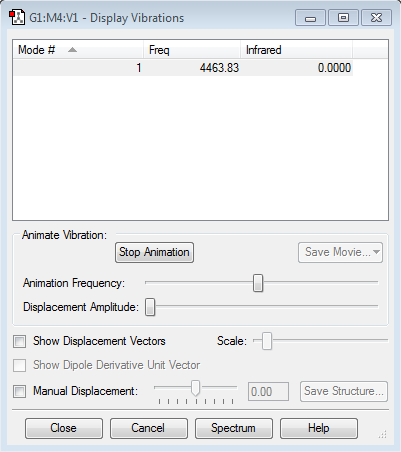
"Display Vibrations" Window
1 Mode of Vibration, no negative frequencies. The vibration is IR inactive as there is no change in dipole moment (molecule is homo-nuclear diatomic).
Reaction Energies
E(NH3)= -56.55776873 a.u.
2*(NH3)= -113.11553746 a.u.
E(N2)= -109.52412868 a.u
E(H2)= -1.17853935 a.u.
3*(H2)= -3.53561805 a.u.
ΔE=2*(NH3)-[E(N2)+3*(H2)]= -113.11553746-[-113.05974673] = -0.05579073 a.u.
ΔE (kJ/mol)= -0.05579073*2625.5 =-146.478561615 kJ/mol
Energy change is negative, therefore the product (Ammonia Gas) is more stable than the reactant gases.
Molecule of Choice - Cyanide CN-
| Optimisation Data | Value |
|---|---|
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d.p) |
| Final Energy (E(RB3LYP)) | -92.82453153 a.u. |
| RMS Gradient | 0.00000704 a.u. |
| Point Group | C∞v |
| C-N Bond Length | 1.18409 Å |
Item Value Threshold Converged?
Maximum Force 0.000012 0.000450 YES
RMS Force 0.000012 0.000300 YES
Maximum Displacement 0.000005 0.001800 YES
RMS Displacement 0.000008 0.001200 YES
Predicted change in Energy=-6.650386D-11
Optimization completed.
-- Stationary point found.
----------------------------
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,2) 1.1841 -DE/DX = 0.0 !
--------------------------------------------------------------------------------
GradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGrad
Cyanide Ion |
The optimisation file is linked to here
"Display Vibrations" Window
1 Mode of Vibration, no negative frequencies. The vibration is IR active, this is because the molecule is a hetero-nuclear diatomic, during vibration there is a change in dipole moment.
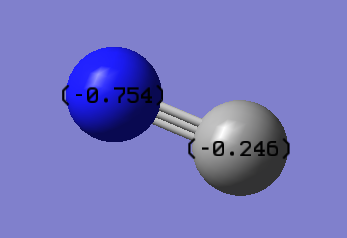
Charge Distribution
| Atom | Charge |
|---|---|
| N | -0.754 |
| C | -0.246 |
Both atoms carry a negative charge as the overall charge of the ion is -1. As nitrogen is more electronegative it has a more negative charge than the carbon.
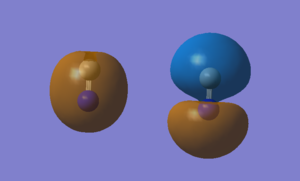
Molecular Orbitals
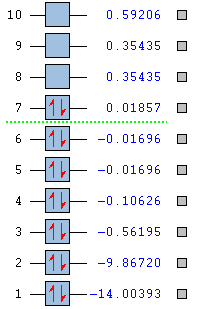
Literature Comparison
The calculated value of the CN bond length is 1.18409 Å, this compares well to a literature value of 1.1554 Å[1], the calculated value is slightly larger, but not by a dramatic amount.
References
- ↑ Tables of Interatomic Distances and Configuration in Molecules and Ions, L.E. Sutton, ed., London: The Chemical Society, 1958.