IMM2 01333017
NH3
Calculation Method: RB3LYP
Basis Set: 6-31G(d,p)
Final Energy:-56.55776873
Point Group: C3v
HNH Bond Angle : 105.741
NH Bond Length:1.01798
Item Value Threshold Converged?
Maximum Force 0.000004 0.000450 YES
RMS Force 0.000004 0.000300 YES
Maximum Displacement 0.000072 0.001800 YES
RMS Displacement 0.000035 0.001200 YES
Predicted change in Energy=-5.986301D-10
Optimization completed.
-- Stationary point found.
NH3 |
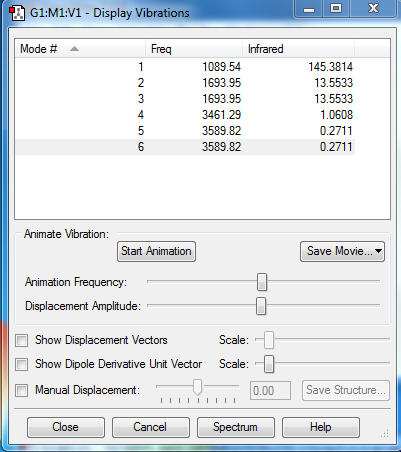
From the 3N-6 rule there are 6 expected vibrational modes. The two asymmetric bending modes are degenerate (2 and 3), as are two asymmetric stretches (5 and 6). 1 is a bending (umbrella) vibration and 4 is a symmetric stretch. Given the degeneracy and symmetric stretches there would be 2 bands in an experimental spectrum of gaseous ammonia.
The nitrogen's charge was calculated to be -1.123 and the hydrogens' +0.375. Since nitrogen is more electronegative than hydrogen it is expected that it would have a negative charge, and the hydrogens positive. The overall molecule should be neutral. Which are all consistent with the calculations.
N2
Calculation Method: RB3LYP
Basis Set: 6-31G(d,p)
Final Energy:-109.52412868
Point Group: D∞h
Item Value Threshold Converged?
Maximum Force 0.000001 0.000450 YES
RMS Force 0.000001 0.000300 YES
Maximum Displacement 0.000000 0.001800 YES
RMS Displacement 0.000000 0.001200 YES
Predicted change in Energy=-3.401001D-13
Optimization completed.
-- Stationary point found.
N2 |
H2
Calculation Method: RB3LYP
Basis Set: 6-31G(d,p)
Final Energy:-1.17853936
Point Group: D∞h
Item Value Threshold Converged?
Maximum Force 0.000000 0.000450 YES
RMS Force 0.000000 0.000300 YES
Maximum Displacement 0.000000 0.001800 YES
RMS Displacement 0.000001 0.001200 YES
Predicted change in Energy=-1.164080D-13
Optimization completed.
-- Stationary point found.
H2 |
Reactivity
E(NH3)= -56.55776873 2*E(NH3)= -113.1155376 E(N2)= -109.52412868 E(H2)= -1.17853936 3*E(H2)= -3.53561808 ΔE=2*E(NH3)-[E(N2)+3*E(H2)]= -0.05579092
-146.47 kJ/mol
As the energy change is negative, ammonia is more stable than the reactants.
S2
Singlet
Calculation Method: RB3LYP
Basis Set: 6-31G(d,p)
Final Energy: -796.32599779
Point Group: D∞h
Item Value Threshold Converged?
Maximum Force 0.000006 0.000450 YES
RMS Force 0.000006 0.000300 YES
Maximum Displacement 0.000011 0.001800 YES
RMS Displacement 0.000016 0.001200 YES
Predicted change in Energy=-7.077694D-11
Optimization completed.
-- Stationary point found.
S2 |
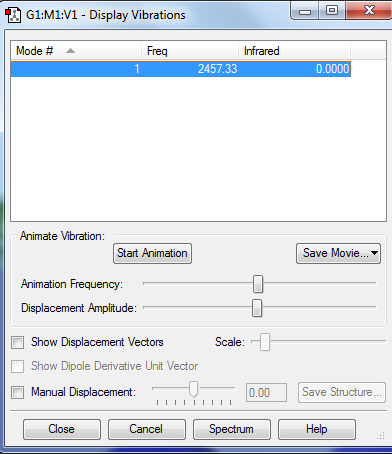
There is only one vibrational mode as the molecule is linear and can therefore only vibrate symmetrically, so there is also no infrared activity.
Since the diatomic molecule is homonuclear there are no charges.
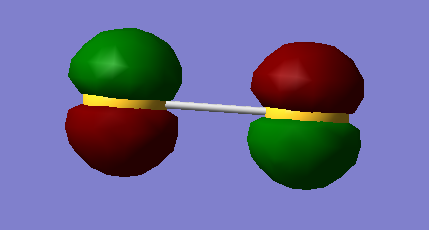
When run as a singlet - suggests the HOMO to be a doubly occupied π* formed from 3p overlap, and the LUMO to be the orthogonal π*. GaussView calculates the π and π* orbitals from 3p AOs to all be of different energies i.e. no degeneracy is shown.
HOMO/LUMO 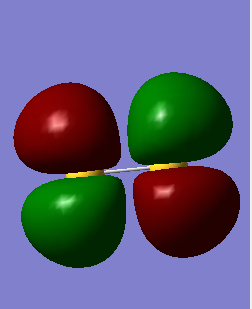
For the deep in energy orbitals - from 2p overlap - the orbitals are so small and contracted they effectively do not interact at all, hence GaussView is unable to assign accurate values.
This leads to a calculated (increasing) energy ordering, for the non-bonding 2p interactions, of 2py in phase, 2py out of phase, 2px in phase, 2px out of phase, 2pz in phase, 2pz out of phase.
Highest Energy (↑y,→x)
Triplet
Calculation Method: RB3LYP
Basis Set: 6-31G(d,p)
Final Energy: -796.36288994
Point Group: D∞h
Item Value Threshold Converged?
Maximum Force 0.000006 0.000450 YES
RMS Force 0.000006 0.000300 YES
Maximum Displacement 0.000010 0.001800 YES
RMS Displacement 0.000014 0.001200 YES
Predicted change in Energy=-5.867929D-11
Optimization completed.
-- Stationary point found.
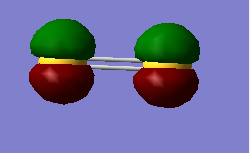
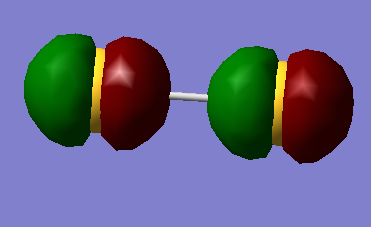
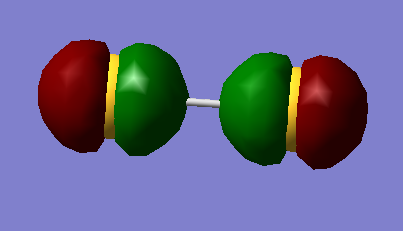
Running calculations again as a triplet yields a different ordering of deep energy orbitals and gives expected degeneracy.
In increasing energy the 2p ordering is given as end-on-end in phase, end-on-end out of phase, two degenerate sideways in phase, two degenerate sideways out of phase.
The HOMO is the two degenerate π* orbitals , which are both singly occupied, so S2 is paramagnetic.
HOMO 3pπg* 
The is HOMO is singly occupied so reduces the bond order, by counteracting to an extent,but not completely, the energy gain from the filled 3pπu orbitals. As the LUMO is unoccupied it makes no contributions to bonding except that in conjunction with the incompletely filled HOMO means the molecule has overall bonding interactions.
These orbitals overall makes no contribution to bonding as they are both fully occupied and energy gain from the σg bonding orbital is cancelled out by the σu* antibonding orbital.
Triplet→Singlet Transition
S2 is a violet gas. Assuming the same cause of coloring in S2 as in liquid oxygen the S2 triplet-singlet energy difference should correspond to absorption in the red/near infrared wavelengths.
Singlet energy= -796.32599779
Triplet energy= -796.36288994
ΔE = +0.03689215 au
= +96.86 kJ/mol
energy per molecule = 1.61 x 10-19 J
= 1.00 eV
energy per photon at 700 nm = 1.77 eV
Absorption for the calculated energies occurs just below visible red so could potentially lead to a higher perceived purple/violet saturation and hence the violet color.