IMM2:Iman Ilyas
CID:01333599
A Molecule of NH3
File:IMAN ILYAS NH3 OPTIMISATION POP 2.log
test molecule |
| Property Of Molecule | Value/Classification |
|---|---|
| Method of Calculation | RB3LYP |
| Basis Set | 6-31G(d,p) |
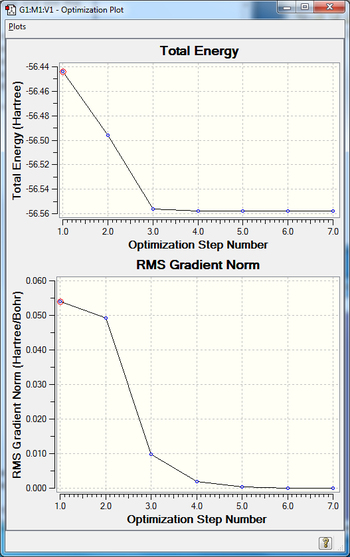
| Final Energy | -56.55776873 |
| The Point Group of NH3 | C3V |
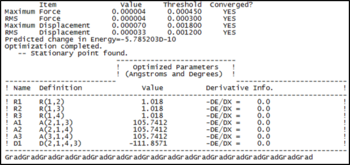
| Bond Length | 1.01798 Angstroms |
| Bond Angle | 105.741 Degrees |
Vibrational Analysis
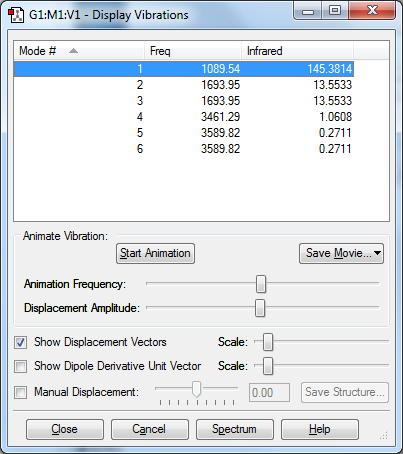
This shows the frequency of different possible vibrations of Ammonia. Using the 3N-6 rule, we would expect 6 modes of vibrations, as 3(4) - 6 = 6. 2 and 3 are degenerate. 5 and 6 are degenerate since they have the same frequency and frequency is proportional to energy as E = hv. 1 and 4 are both highly symmetric vibrations. Vibration 1 is the umbrella mode. We expect to see 4 bands in an experimental spectrum as there are 6 vibrational modes, of which 2 sets are degenerate.
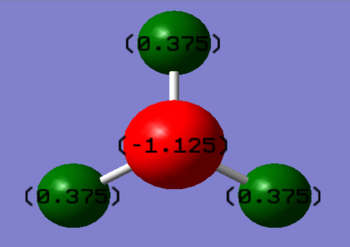
Charge Analysis
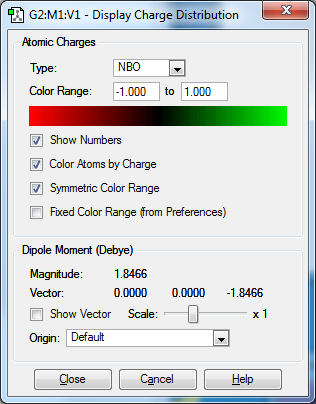
I would expect a negative charge on the Nitrogen since it is more electronegative than the Hydrogens. This means it strongly pulls the pair of electrons in the covalent bonds towards itself.
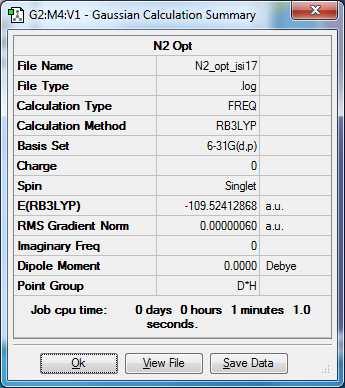
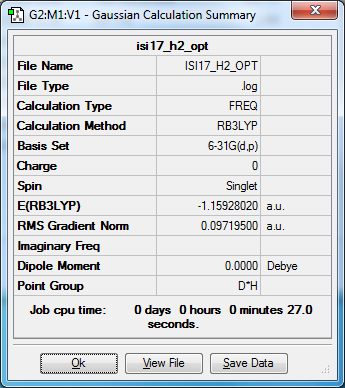
N2 and H2
E(NH3)= -56.55776873 2*E(NH3)= -113.11553746 E(N2)= -109.52412868 E(H2)= -1.15928020 3*E(H2)= -3.4778406 ΔE=2*E(NH3)-[E(N2)+3*E(H2)]= -0.11356818*2625.5 = -298.17325659 kJ/mol
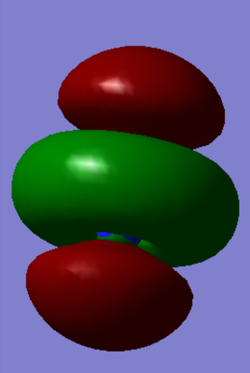
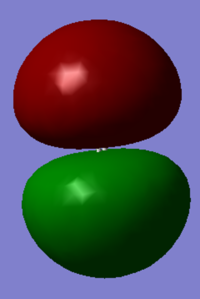
Molecular Orbitals of N2
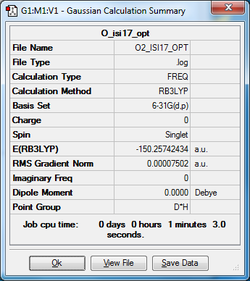
A Molecule of my Choice:O2
test molecule |
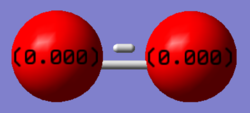
This shows a charge of 0 on both since they are both the same atom (oxygen) and therefore no permanent dipole. This is a neutral molecule with no overall charge with a bond length of 1.21602 Angstroms.