ICL:zl4817
BH3 Molecule
Link: LZY_BH3_OPT_FREQ.LOG
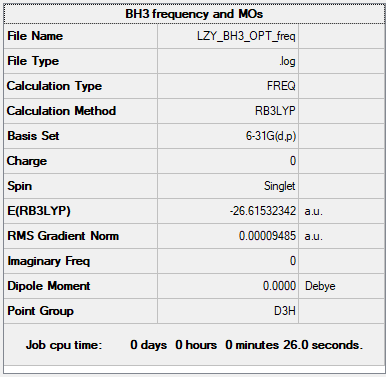
Summary Table
B3LYP/6-31G(d.p) level
Figure 1: Summary Table of BH3
Item Table
Item Value Threshold Converged? Maximum Force 0.000190 0.000450 YES RMS Force 0.000095 0.000300 YES Maximum Displacement 0.000747 0.001800 YES RMS Displacement 0.000374 0.001200 YES
Frequency Analysis
Low frequencies --- -0.2260 -0.1035 -0.0054 48.0331 49.0927 49.0932 Low frequencies --- 1163.7226 1213.6716 1213.6743
Jmol Dynamic Image
BH3 |
Table of Vibrational Modes
| Wavenumber (cm-1 | Intensity (a.u.) | Symmetry Point Group | IR active? | Vibrational Mode |
| 1163 | 92 | A2'' | yes | out-of-plane bend |
| 1213 | 14 | E' | yes | bend |
| 1213 | 14 | E' | yes | bend |
| 2580 | 0 | A1' | no | symmetric stretch |
| 2713 | 126 | E' | yes | asymmetric stretch |
| 2713 | 126 | E' | yes | asymmetric stretch |
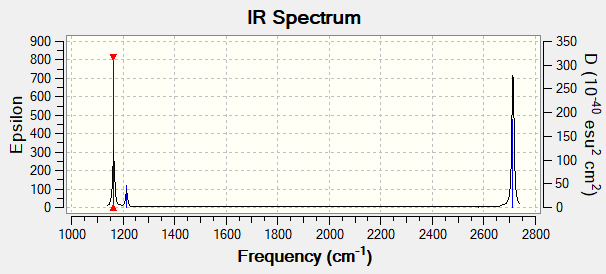
IR Spectrum
Figure 2: BH3 Proposed IR Spectrum by Gaussian
Explanation for Number of Peaks in Spectrum
There are two sets of degenerate vibrations at 1213 cm-1 (bend) and 2713 cm-1 (asymmetric stretch). A maximum of 4 signals will appear on the spectrum.
Moreover, the vibration mode at 2580 cm-1 is a symmetric stretch, which is IR inactive.
Hence only three signals are found in the IR spectrum.
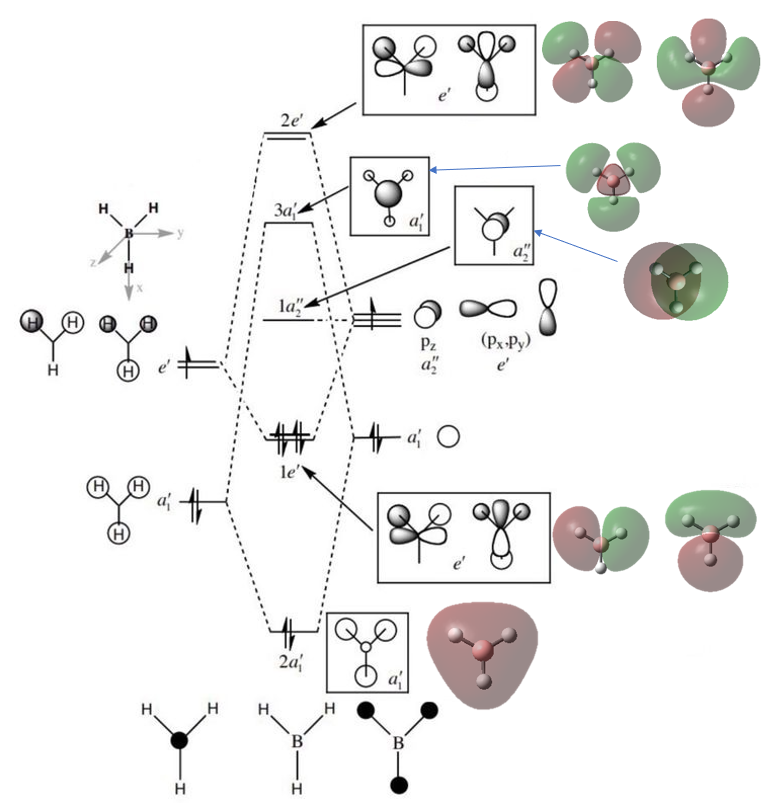
MO Diagram of BH3
Figure 3: BH3 LCAO and 'Real' MO Diagram
The MOs of BH3 produced by LCAO (black and white) and MOs produced by Gaussian Calculation (coloured) are shown in the above diagram.[1]
There are some small differences between the LCAO MO diagram and the 'real' diagrams performed by Gaussian.
- In-phase orbitals in 'real' MOs merge together to form a joint orbital. However, individual orbitals in the LCAO diagram remain separate.
Ng611 (talk) 21:12, 5 June 2019 (BST) How significant do you think this is?
- The 'real' MOs accounts for repulsion between orbital, which are shown by slightly bent p orbitals.
Ng611 (talk) 21:12, 5 June 2019 (BST) Yes although we sometimes mark orbitals as being 'polarised' off atomic centres.
Comparing the results, we may conclude that the LCAO diagram shows the 'real' spatial electron density distribution in a relatively accurate manner.
NH3 Molecule
Link: LZY_NH3_FREQ_2.LOG
Ng611 (talk) 21:13, 5 June 2019 (BST) This is your opt+freq file, not your freq .log file.
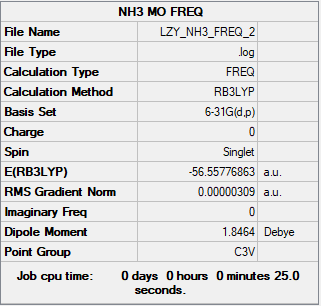
Summary Table
B3LYP/6-31G(d.p) level
Figure 4: NH3 Summary Table
Item Table
Item Value Threshold Converged? Maximum Force 0.000006 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000013 0.001800 YES RMS Displacement 0.000008 0.001200 YES
Frequency Analysis
Low frequencies --- -11.3135 -11.2772 -0.0036 0.0250 0.1442 25.7141 Low frequencies --- 1089.6627 1694.1740 1694.1743
Jmol Dynamic Image
NH3 |
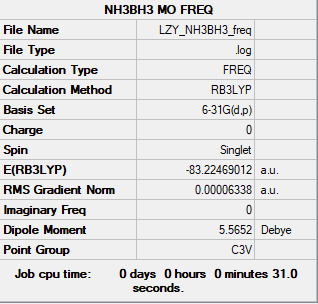
NH3BH3 Molecule
Link: LZY_NH3BH3_FREQ.LOG
Summary Table
B3LYP/6-31G(d.p) level
Figure 5: NH3BH3 Summary Table
Item Table
Item Value Threshold Converged? Maximum Force 0.000113 0.000450 YES RMS Force 0.000063 0.000300 YES Maximum Displacement 0.000616 0.001800 YES RMS Displacement 0.000354 0.001200 YES
Frequency Analysis
Low frequencies --- -0.0615 -0.0457 -0.0066 21.6949 21.7008 40.5895 Low frequencies --- 266.0361 632.3691 640.1428
Jmol Dynamic Image
NH3BH3 |
Association Energy Analysis
E(NH3)= -56.55777 au
E(BH3)= -26.61532 au
E(NH3BH3)= -83.22469 au
ΔE=E(NH3BH3)-[E(NH3)+E(BH3)] = -0.05160 au = -0.05160 ÷ 0.0004 kJ/mol = -129 kJ/mol (1 kJ/mol = 0.0004 au) [2]
Ng611 (talk) 21:14, 5 June 2019 (BST) You've used the wrong conversion factor (1 kJ/mol = 0.00038 a.u.) and your final answer is off by a few kJ/mol. Otherwise, great calculation.
Hence, N-B dative bond energy is -129 kJ/mol.
It is much weaker than a normal N-B sigma bond (-377.9 kJ/mol).[3]
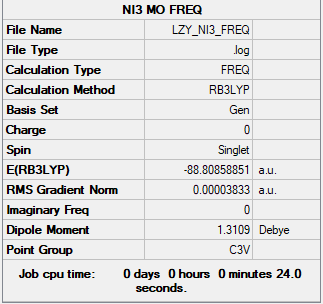
NI3 Molecule
Link: LZY_NI3_FREQ.LOG
Summary Table
B3LYP/Gen level
Figure 6: NI3 Summary Table
Item Table
Item Value Threshold Converged? Maximum Force 0.000064 0.000450 YES RMS Force 0.000038 0.000300 YES Maximum Displacement 0.000486 0.001800 YES RMS Displacement 0.000277 0.001200 YES
Frequency Analysis
Low frequencies --- -12.7375 -12.7314 -6.2899 -0.0039 0.0188 0.0633 Low frequencies --- 101.0325 101.0332 147.4122
Jmol Dynamic Image
NI3 |
- N-I bond distance: 2.184 Å
Project: Lewis Acids and Bases
Different Al2Cl4Br2 Isomers
Isomer A
Ng611 (talk) 21:17, 5 June 2019 (BST) You've forgotten to use pseudopotentials in all of you calculations in this section.
Link: LZY_ISOMER_A_2.LOG
Molecular Diagram
Figure 7: Isomer A
Summary Table
B3LYP/6-31G(d.p)
Figure 8: Isomer A Summary Table
Item Table
Item Value Threshold Converged? Maximum Force 0.000200 0.000450 YES RMS Force 0.000094 0.000300 YES Maximum Displacement 0.000826 0.001800 YES RMS Displacement 0.000375 0.001200 YES
Frequency Analysis
Low frequencies --- -2.7897 -2.7222 -0.0167 0.0065 0.0076 3.3444 Low frequencies --- 11.5302 65.3796 88.6180
Jmol Dynamic Image
ISOMER A |
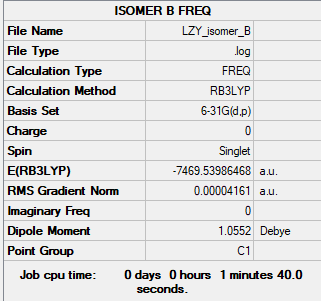
Isomer B
Link: LZY_ISOMER_B.LOG
Molecular Diagram
Figure 9: Isomer B
Summary Table
B3LYP/6-31G(d.p)
Figure 10: Isomer B Summary Table
Item Table
Item Value Threshold Converged? Maximum Force 0.000080 0.000450 YES RMS Force 0.000031 0.000300 YES Maximum Displacement 0.000744 0.001800 YES RMS Displacement 0.000314 0.001200 YES
Frequency Analysis
Low frequencies --- -3.7545 -0.0084 -0.0076 0.0055 2.9743 5.3976 Low frequencies --- 15.0735 58.8033 81.4340
Jmol Dynamic Image
ISOMER B |
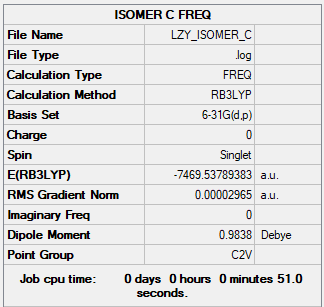
Isomer C
Link: LZY_ISOMER_C_1.LOG
Molecular Diagram
Figure 11: Isomer C
Summary Table
B3LYP/6-31G(d.p)
Figure 12: Isomer C Summary Table
Item Table
Item Value Threshold Converged? Maximum Force 0.000085 0.000450 YES RMS Force 0.000030 0.000300 YES Maximum Displacement 0.000753 0.001800 YES RMS Displacement 0.000244 0.001200 YES
Frequency Analysis
Low frequencies --- -1.8103 -0.0157 -0.0134 -0.0124 3.1304 3.1724 Low frequencies --- 13.1236 54.3351 73.8370
Jmol Dynamic Image
ISOMER C |
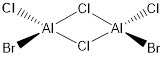
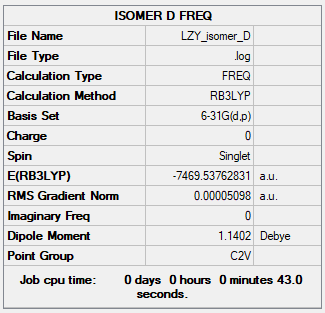
Isomer D
Link: LZY_ISOMER_D.LOG
Molecular Diagram
Figure 13: Isomer D
Summary Table
B3LYP/6-31G(d.p)
Figure 14: Isomer D Summary Table
Item Table
Item Value Threshold Converged? Maximum Force 0.000096 0.000450 YES RMS Force 0.000029 0.000300 YES Maximum Displacement 0.001637 0.001800 YES RMS Displacement 0.000724 0.001200 YES
Frequency Analysis
Low frequencies --- -8.9580 -3.9751 -3.5921 -0.0122 -0.0093 -0.0075 Low frequencies --- 16.2873 53.6939 79.7829
Jmol Dynamic Image
ISOMER D |
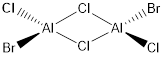
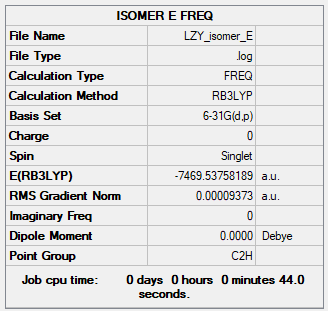
Isomer E
Link: LZY_ISOMER_E.LOG
Molecular Diagram
Figure 15: Isomer E
Summary Table
B3LYP/6-31G(d.p)
Figure 16: Isomer E Summary Table
Item Table
Item Value Threshold Converged? Maximum Force 0.000208 0.000450 YES RMS Force 0.000056 0.000300 YES Maximum Displacement 0.000699 0.001800 YES RMS Displacement 0.000265 0.001200 YES
Frequency Analysis
Low frequencies --- -4.5056 -2.1615 0.0048 0.0063 0.0067 3.1231 Low frequencies --- 15.1154 52.1657 73.8402
Jmol Dynamic Image
ISOMER E |
Symmetry and Energy of Al2Cl4Br2 Isomers
Observations
When both bridging ions are Br, the molecule has highest stability.
The stability decreases when one of the bridging ions is replaced by Cl, and is lowest when both of the bridging ions are Cl.
Explanations
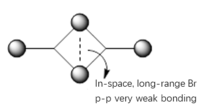
The Al-X-Al bridging bond (X = Br or Cl) consists of a σ-type bond, and a dative bond with X as the electron donor (as shown in the figure below).
The strength of the Al-X-Al bond is proportional to the orbital overlap efficiency between Al and X, and the bond strength is in turn proportional to the relative stability of the complex molecule. [4]
Br is lower in Group 17, hence it has larger and more diffuse orbitals, making the orbital overlap with Al more efficient. In contrast, Cl is higher in Group 17 and it is very electronegative. Hence, it has a smaller radius and its nucleus holds its electrons tightly.
Therefore, we may conclude the Al-Br-Al bonding provides greater stability than the Al-Cl-Al one.
Ng611 (talk) 21:22, 5 June 2019 (BST) Because of your incorrect pseudopotential input, you've (incorrectly) calculated that the D2h (bridging Br) structure is more stable. In fact, the opposite is true.
Figure 17: A General Structure of Dimer Complex
Dissociation of the Lowest-Energy Conformer into 2 AlCl2Br
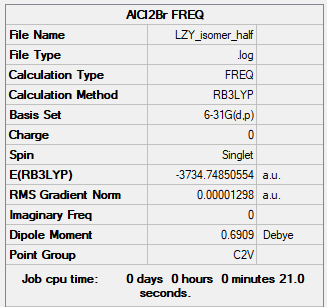
Link: LZY_ISOMER_HALF_1.LOG
Summary Table
B3LYP/6-31G(d.p)
Figure 18: AlCl2Br Summary Table
Item Table
Item Value Threshold Converged? Maximum Force 0.000028 0.000450 YES RMS Force 0.000013 0.000300 YES Maximum Displacement 0.000083 0.001800 YES RMS Displacement 0.000048 0.001200 YES
Frequency Analysis
Low frequencies --- -3.3374 -1.9339 0.0130 0.0138 0.0142 4.7835 Low frequencies --- 125.0015 137.4966 194.8482
Jmol Dynamic Image
AlCl2Br |
Comparison of Energy
Isomer A is the conformer with the lowest energy.
When the dimer dissociates into two molecules of AlCl2Br, 2 Al-Br bonds are lost.
The energy of one molecule of AlCl2Br is calculated to be -9336871 kJ/mol by Gaussian.
Hence, the sum of energy of two AlCl2Br molecules is -18673743 kJ/mol which is 114 kJ/mol higher relative to the energy of Isomer A.
As breaking bonds involves absorption of energy[5], each Al-Br bond broken increases total energy by 57 kJ/mol.
This explains why the dimer is more stable than the dissociated monomers.
Ng611 (talk) 21:23, 5 June 2019 (BST) e.c.f for calculation.
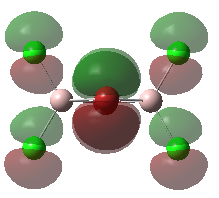
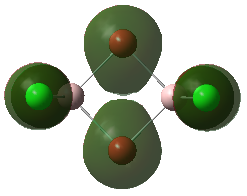
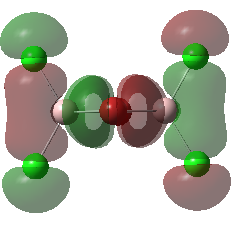
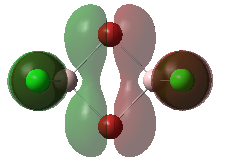
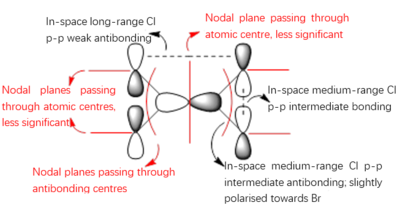
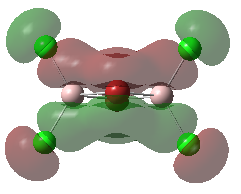
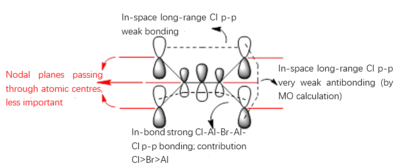
Molecular Orbitals of the Conformer with Lowest Energy
The lowest-energy conformer is Isomer A. There are 24 occupied valence molecular orbitals in total.
Three among them are selected, analysed and arranged in order by their extent of bonding/antibonding character.
Atom colour label: pink - Al, green - Cl, red - Br
Ng611 (talk) 21:25, 5 June 2019 (BST) Excellent LCAO analysis! Very neatly laid-out and well annontated. Well done!
References
- ↑ http://www.huntresearchgroup.org.uk/teaching/teaching_comp_lab_year2a/Tut_MO_diagram_BH3.pdf .
- ↑ http://www.huntresearchgroup.org.uk/teaching/teaching_comp_lab_year2a/4b_better_basis.html .
- ↑ E.R. Cohen, T. Cvitas, J.G. Frey, B. Holmström, K. Kuchitsu, R. Marquardt, I. Mills, F. Pavese, M. Quack, J. Stohner, H.L. Strauss, M. Takami, and A.J. Thor, Quantities, Units and Symbols in Physical Chemistry, IUPAC Green Book, Third Edition, Second Printing, IUPAC & RSC Publishing, Cambridge (2008)
- ↑ C. Barnes, Inorganic Chemistry (Housecroft, Catherine E.; Sharpe, Alan G.), J. Chem. Educ., 2003, 80, 747.
- ↑ Peter Atkins and Julio de Paula, Physical Chemistry (8th ed., W.H. Freeman 2006), p.809 ISBN 0-7167-8759-8