ICL:1905
Exercise 1: Borane - BH3
B3LYP/3-21G summary
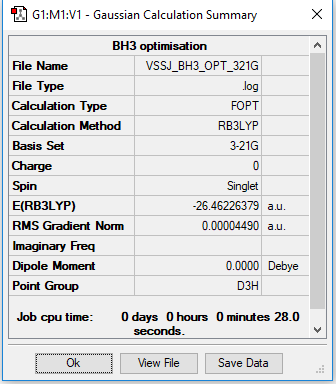
Ng611 (talk) 15:48, 5 June 2019 (BST) You should include the summary table for the frequency analysis .log file you provide.
Item Value Threshold Converged?
Maximum Force 0.000090 0.000450 YES
RMS Force 0.000059 0.000300 YES
Maximum Displacement 0.000350 0.001800 YES
RMS Displacement 0.000229 0.001200 YES
Predicted change in Energy=-4.546985D-08
Optimization completed.
-- Stationary point found.
Low frequencies --- -6.8246 -1.4177 -0.0054 0.7248 7.6960 7.8255 Low frequencies --- 1162.9713 1213.1658 1213.1685
Frequency analysis log file: VSSJ_BH3_freq.log
Borane |
The optimised B-H bond length is 1.19231 Å.
Vibrational spectrum for BH3
| Wavenumber (cm-1 | Intensity (arbitrary units) | Symmetry | IR active? | Type of vibration |
|---|---|---|---|---|
| 1162.97 | 93 | A2" | Yes | Out-of-plane bending. |
| 1213.17 | 14 | E' | Yes | Asymmetric bending (of 2 B-H bonds) |
| 1213.17 | 14 | E' | Yes | Asymmetric bending |
| 2582.36 | 0 | A1' | No | Symmetric stretching |
| 2715.54 | 126 | E' | Yes | Asymmetric stretching (of 2 B-H bonds) |
| 2715.54 | 126 | E' | Yes | Asymmetric stretching |
Ng611 (talk) 15:49, 5 June 2019 (BST) Your 2x modes at 1213 cm-1 are degenerate and therefore should have identical assignments. From your comment on the type of vibration, it makes me think that you think they're not.
BH3 IR Spectrum
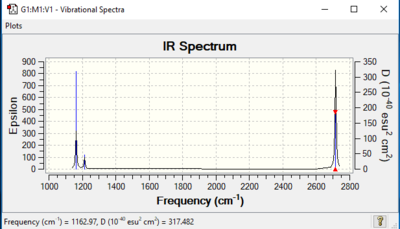
Despite there obviously being 6 vibrations, only 3 are visible in borane's infra-red spectrum. This is because there are vibrations that correspond to the same wavenumber, and therefore appear as 1 intensified peak. For example, there are 2 different asymmetric bends in borane; however since they have the same wavenumber, intensity, symmetry and are both IR active, they appear as 1 peak. The same goes with the 2 different asymmetric stretches. Lastly, the vibration corresponding to v=2582.36 cm-1 is not visible on the spectrum because the symmetric stretches cancel each other out, resulting in no net dipole moment and therefore is IR inactive.
Ng611 (talk) 15:52, 5 June 2019 (BST) Can you see any differences at all?
Molecular Orbital Diagram of BH3
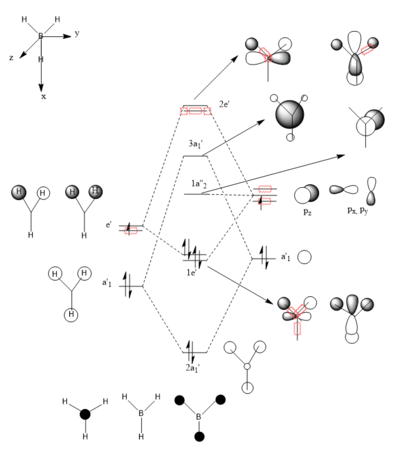
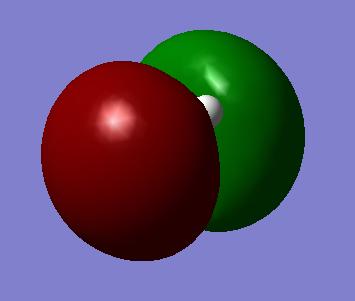
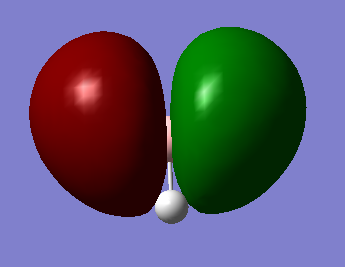

Ng611 (talk) 15:51, 5 June 2019 (BST) Two orbitals (your 3a1 orbital and one of your 2e' orbitals) are missing here
Are there any significant differences between the real and LCAO MOs?
The only significant difference observed between the real and LCAO MOs is that the MO diagram shows the atomic orbitals as they are before combination occurs, while the MOs from Gaussview have already combined them.
What does this say about the accuracy and usefulness of qualitative MO theory?
It is highly accurate and useful.
Exercise 2: Ammonia-Borane
Ng611 (talk) 15:53, 5 June 2019 (BST) Where are the results for the NH3 and BH3NH£ adduct calculations?
E(NH3)= -56.55776873 au, E(BH3)= -26.61532364 au, E(NH3BH3)= -83.22468893 a.u.
ΔE=E(NH3BH3)-[E(NH3)+E(BH3)] = -83.22468893 - (-56.55776873 - 26.61532364) = -0.05159656 a.u. = -135.47 kJ mol-1
Ng611 (talk) 15:54, 5 June 2019 (BST) Too many d.p. Your answers are correct to ~1 kJ/mol and your final answer should be reported as such
Looking at this obtained value for the N-B dative covalent bond strength, it is comparatively weak when also looking at N-N (167 kJ mol-1) and B-B (293 kJ mol-1). This might be because it is not a fully covalent bond, and the reason for assuming it's a weak bond is because the N-N bond is weak too (due to repulsion of the lone pairs surrounding N, leaving the single bond to be quite unstable), and yet the strength of the N-N bond is greater than the N-B bond.
Ng611 (talk) 15:54, 5 June 2019 (BST) Where's your reference for these values?!
Exercise 3: Nitrogen Triiodide - NI3
Frequency analysis log file: VSSJ_NI3_FREQ.log
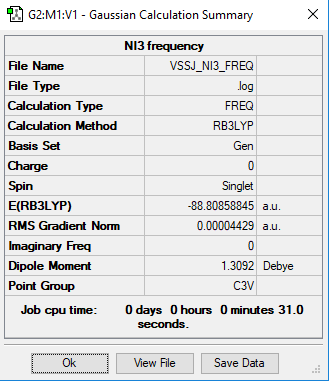
Item Value Threshold Converged?
Maximum Force 0.000088 0.000450 YES
RMS Force 0.000044 0.000300 YES
Maximum Displacement 0.000858 0.001800 YES
RMS Displacement 0.000481 0.001200 YES
Predicted change in Energy=-1.191918D-07
Optimization completed.
-- Stationary point found.
Low frequencies --- -12.3847 -12.3783 -5.6131 -0.0040 0.0194 0.0711 Low frequencies --- 100.9307 100.9314 147.2333
Nitrogen triiodide |
The optimised N-I distance is 2.18424 Å.
Ng611 (talk) 15:55, 5 June 2019 (BST) Again, too many d.p.
Mini project - Lewis Acids and Bases - Al2Cl4Br2
Al2Cl4Br2 where Br atoms are trans and Cl atoms are bridging Al atoms together
Frequency analysis log file: VSSJ_AL2CL4BR2_FREQ.log
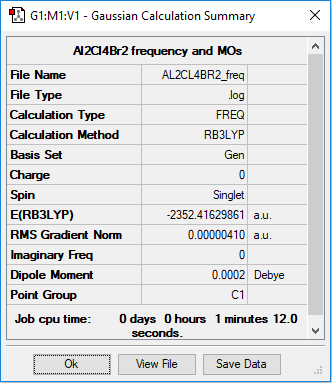
Item Value Threshold Converged?
Maximum Force 0.000011 0.000450 YES
RMS Force 0.000004 0.000300 YES
Maximum Displacement 0.000439 0.001800 YES
RMS Displacement 0.000151 0.001200 YES
Predicted change in Energy=-3.294505D-09
Optimization completed.
-- Stationary point found.
Low frequencies --- -5.1504 0.0023 0.0023 0.0038 1.4135 2.0504 Low frequencies --- 18.1470 49.1065 73.0086
Dialuminium tetrachloride dibromide (trans) |
The optimised Al-Br bond length is 2.27463 Å. The optimised Al-tCl bond length is 2.09378 Å. The optimised Al-μCl bond lengths are: 2.29812 Å and 2.29803 Å.
Al2Cl4Br2 where Br atoms are bridging Al atoms together
Frequency analysis log file: VSSJ_AL_BR_BRIDGING_OPT.log
Dialuminium tetrachloride dibromide (Br atoms are bridging) |
Ng611 (talk) 16:03, 5 June 2019 (BST) Where are your other structures?
Questions and answers - Al2Cl4Br2
1. Determine the five possible isomers and identify the symmetry of each isomer of Al2Cl4Br2.
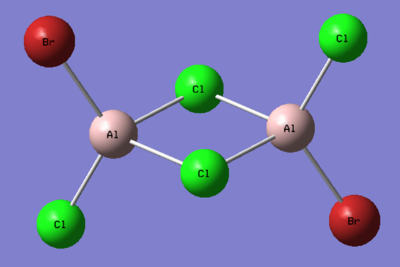
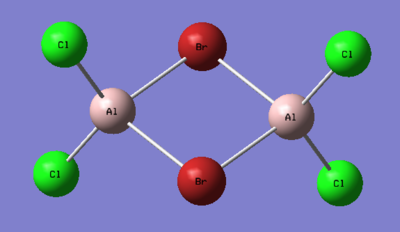
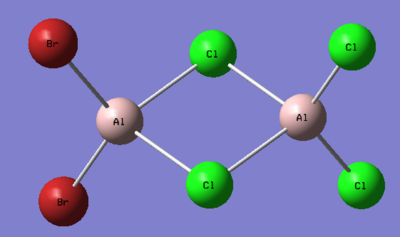

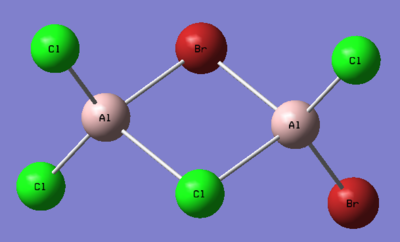
Ng611 (talk) 16:02, 5 June 2019 (BST) These are mostly incorrect. Did you use the point group given to you by gaussian? They aren't always accurate and you should do the point group assignment by hand. If you did, I'd reccomend reviewing your notes.
2. Compute the energy of the isomers with (a) 2 bridging Br ions and (b) the isomer with trans terminal Br and bridging Cl atoms.
(a)- The energy of the isomer with bridging Br atoms is -2352.4063 a.u. (b)- The energy of the isomer with trans terminal Br atoms and bridging Cl atoms is -2352.4163 a.u.
3. Determine the relative energy of these isomers in kJ/mol.
(a)- The energy of the isomer with bridging Br atoms is -6176242 kJ mol-1. (b)- The energy of the isomer with trans terminal Br atoms and bridging Cl atoms is -6176269 kJ mol-1.
4. Discuss the relative stability of these conformers with respect to the bridging ions.
Br is a bigger atom than Cl, therefore the Al-Br bridging bond is longer than that of Al-Cl, which results in poorer orbital overlap between Al and Br. This means that the isomer where Br atoms are bridging is less stable than those where Cl atoms are bridging.
5. Determine the dissociation energy for the lowest energy conformer into 2AlCl2Br.
Dissociation energy => ΔE = E(Al2Cl4Br2) - 2E(AlCl2Br) = -2352.41629861 a.u. - (2 X -1176.79013674 a.u.) = 1.16397487 a.u. ~ 1.1640 a.u. ~ 3056 kJ mol-1
6. Is the product more or less stable than the isolated monomers?
The dimer, i.e. Al2Cl4Br2, is more stable than the isolated monomer, i.e. AlCl2Br. Dimerisation occurs in order to reduce the electron deficiency seen in Al. It is also because the 3p-3p π orbital overlap (between Al and Cl) is not good in AlCl2Br, and greater orbital overlap is seen in the dimer, hence making it more stable.
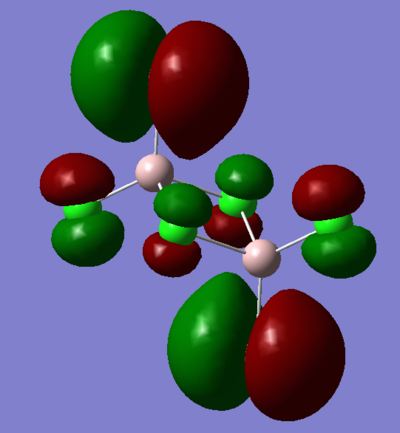
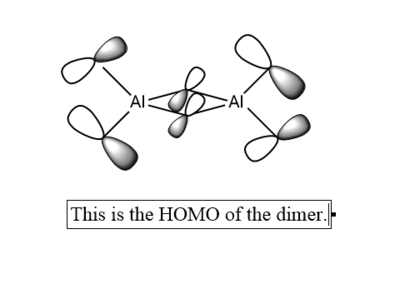
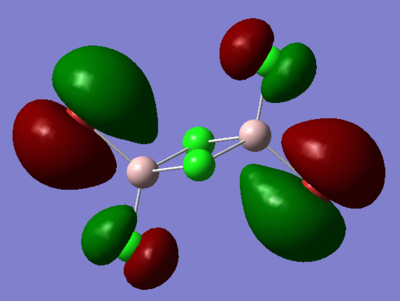
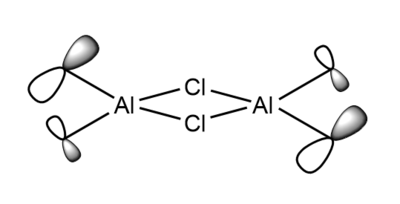
7. Carry out a MO calculation on the lowest energy isomer (only!)
| Real MO | LCAOs |
|---|---|
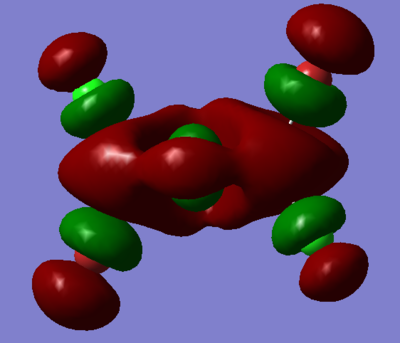 |
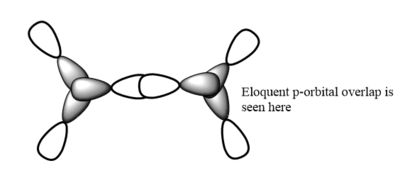
|
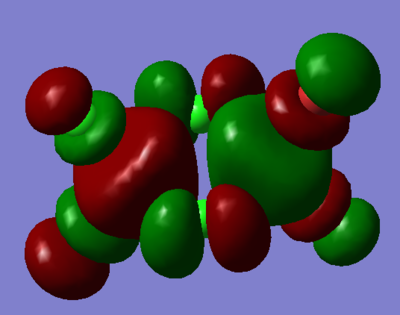 |
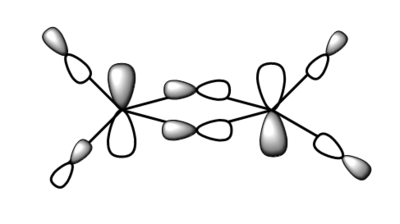
|
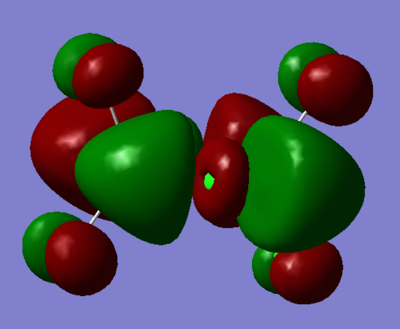 |
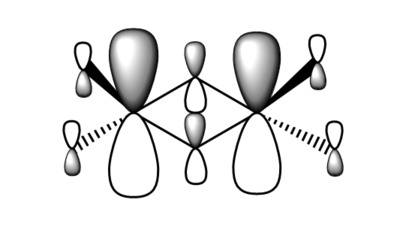
|
 |

|
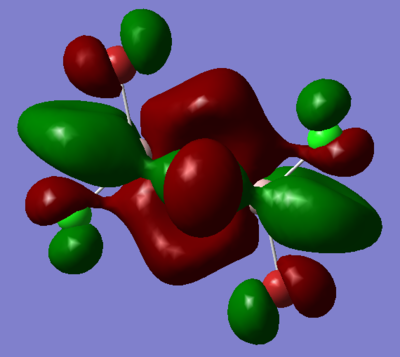 |
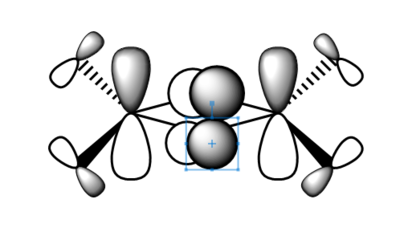
|
 |
|
 |
|
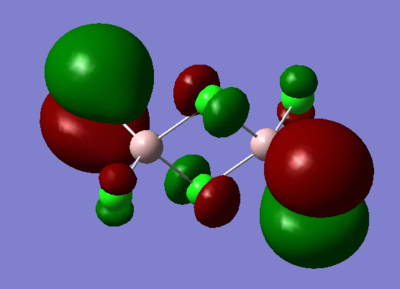 |
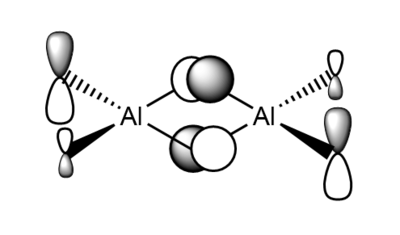
|
Ng611 (talk) 16:11, 5 June 2019 (BST) You only needed to provide 3x orbitals here. Your bottom three look good. You should label the key orbital interactions on your LCAO diagrams.
