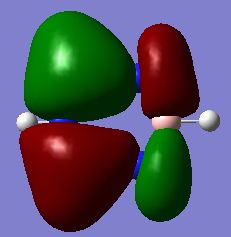ICC:ceh15
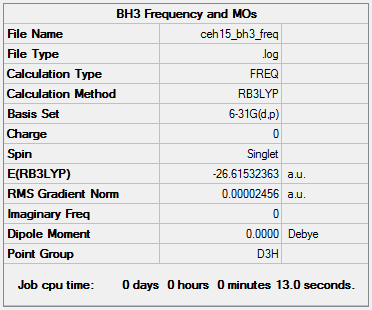
BH3: B3LYP/6-31G level
Item Value Threshold Converged? Maximum Force 0.000049 0.000450 YES RMS Force 0.000025 0.000300 YES Maximum Displacement 0.000193 0.001800 YES RMS Displacement 0.000097 0.001200 YES
Low frequencies --- -0.2279 -0.0080 0.0004 22.0037 22.0049 24.0346 Low frequencies --- 1163.1731 1213.2725 1213.2727
Borane |
Vibrational spectrum for BH3
| wavenumber (cm-1) | Intensity (arbitrary units) | symmetry | IR active? | type |
| 1163 | 92 | A2" | yes | out-of-plane bend |
| 1213 | 14 | E' | very slight | bend |
| 1213 | 14 | E' | very slight | bend |
| 2582 | 0 | A1' | no | symmetric stretch |
| 2715 | 126 | E' | yes | asymmetric stretch |
| 2715 | 126 | E' | yes | asymmetric stretch |
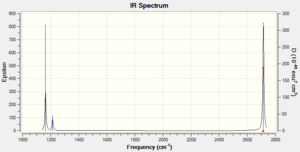
In the spectrum of BH3, three peaks can be seen, however, 6 vibrations are listen in the table calculated by Gaussian. This is because some of the peaks have the same frequency, i.e. are degenerate. However this only accounts for two of the missing peaks. The third peak is missing because it is no IR active, since the dipole moment doesn't change during the vibration. This means that the intensity of this peak is 0 in the table, and it doesn't show up on the spectrum.
Smf115 (talk) 00:16, 26 May 2018 (BST)Correctly assigned vibrational modes and symmetries with a good explaination of why only 3 peaks are seen.
Molectular Orbitals of BH3
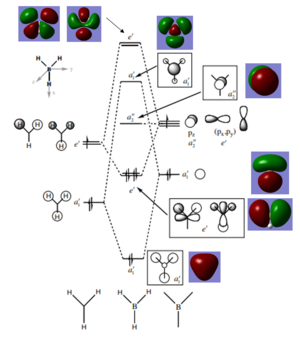
There are not significant differences between the calculated and LCAO molecular orbitals. For this molecule, qualitative molecular orbital theory is sufficient to describe the orbitals and bonding. However, for larger and more complex molecules, it may not be sufficiently accurate.
Smf115 (talk) 00:15, 26 May 2018 (BST)Clear inclusion of the MOs on the diagram and good conclusion to how useful qualitative MO theory is. To improve, it would have been good notice some of the differences between the shapes of the MOs, such as the contributions from the H's in the 3a1' MO.
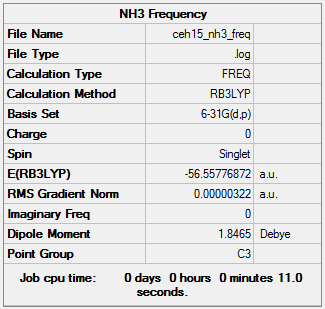
NH3: B3LYP/6-31G level
Item Value Threshold Converged? Maximum Force 0.000006 0.000450 YES RMS Force 0.000003 0.000300 YES Maximum Displacement 0.000013 0.001800 YES RMS Displacement 0.000007 0.001200 YES
Low frequencies --- -0.0137 -0.0026 0.0012 7.0783 8.0932 8.0937 Low frequencies --- 1089.3840 1693.9368 1693.9368
Ammonia |
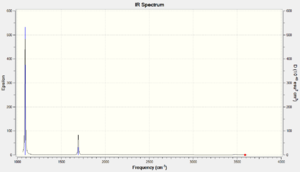
Vibrational spectrum for NH3
| wavenumber (cm-1) | Intensity (arbitrary units) | symmetry | IR active? | type |
| 1089 | 145 | A | yes | out-of-plane bend |
| 1694 | 14 | E | very slight | bend |
| 1694 | 14 | E | very slight | bend |
| 3461 | 1 | A | very slight | symmetric stretch |
| 3590 | 0 | E | no | asymmetric stretch |
| 3590 | 0 | E | no | asymmetric stretch |
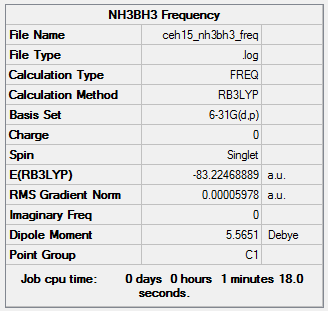
NH3BH3: B3LYP/6-31G level
Item Value Threshold Converged? Maximum Force 0.000116 0.000450 YES RMS Force 0.000060 0.000300 YES Maximum Displacement 0.000579 0.001800 YES RMS Displacement 0.000346 0.001200 YES
Low frequencies --- -0.0017 -0.0014 -0.0010 16.2531 17.3981 37.2581 Low frequencies --- 265.8400 632.1980 639.2687
Ammonia-Borane Complex |
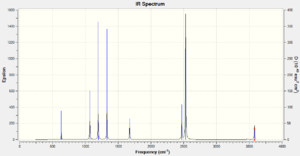
Vibrational spectrum for NH3BH3
| wavenumber (cm-1) | Intensity (arbitrary units) | symmetry | IR active? | type |
| 266 | 0 | A | no | torsion |
| 632 | 14 | A | very slight | symmetric stretch |
| 639 | 4 | A | very slight | bend |
| 639 | 4 | A | very slight | bend |
| 1069 | 40 | A | yes | bend |
| 1069 | 40 | A | yes | bend |
| 1196 | 109 | A | yes | borane out of plane bend |
| 1203 | 3 | A | very slight | borane bend |
| 1203 | 3 | A | very slight | borane bend |
| 1329 | 114 | A | yes | ammonia out of plane bend |
| 1676 | 28 | A | yes | ammonia bend |
| 1676 | 28 | A | yes | ammonia bend |
| 2470 | 67 | A | yes | borane symmetric stretch |
| 2530 | 231 | A | yes | borane asymmetric stretch |
| 2530 | 231 | A | yes | borane asymmetric strech |
| 3463 | 2 | A | very slight | ammonia symmetric stretch |
| 3579 | 28 | A | yes | ammonia asymmetric stretch |
| 3579 | 28 | A | yes | ammonia asymmetric stretch |
Ammonia-Borane Association Energies
| Molecule | Energy /a.u. |
| NH3 | -56.55777 |
| BH3 | -26.61532 |
| NH3BH3 | -83.22469 |
ΔE = E(NH3BH3)-[E(NH3)+E(BH3)] = -0.05160 a.u. = -129 kJ.mol-1
The B-N dative bond is reasonably weak, when compared with the C-C single bond, which has an energy of 346 kJ.mol-1.[2]
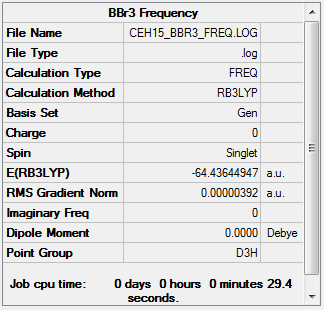
BBr3: B3LYP/6-31G(d,p) LanL2DZ level
Item Value Threshold Converged? Maximum Force 0.000008 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000037 0.001800 YES RMS Displacement 0.000018 0.001200 YES
Low frequencies --- -2.3055 -0.0029 -0.0018 0.0774 0.7534 0.7534 Low frequencies --- 155.9402 155.9405 267.6894
BBr |
Aromaticity Project
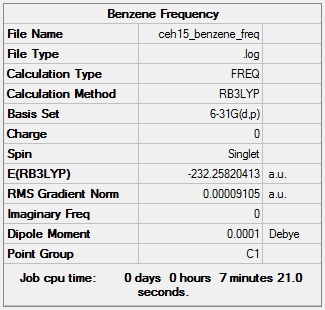
Benzene Key Information: B3LYP/6-31G level
Item Value Threshold Converged? Maximum Force 0.000187 0.000450 YES RMS Force 0.000091 0.000300 YES Maximum Displacement 0.000823 0.001800 YES RMS Displacement 0.000358 0.001200 YES
Low frequencies --- -13.7810 -12.9763 -11.9029 -0.0003 0.0003 0.0005 Low frequencies --- 414.0699 414.1914 620.9703
Benzene |
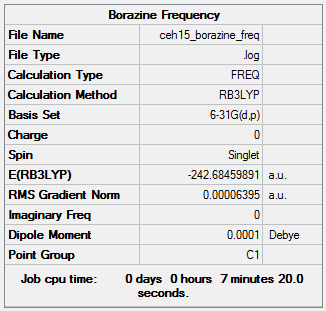
Borazine Key Information: B3LYP/6-31G level
Item Value Threshold Converged? Maximum Force 0.000202 0.000450 YES RMS Force 0.000064 0.000300 YES Maximum Displacement 0.000300 0.001800 YES RMS Displacement 0.000106 0.001200 YES
Low frequencies --- -5.9262 -0.0010 -0.0005 0.0005 5.1016 8.3042 Low frequencies --- 289.5657 289.6523 404.3296
Borazine |
Charge Distribution Analysis
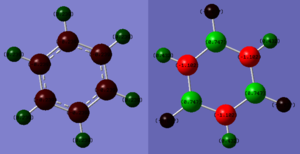
| Atom | Charge |
| C | -0.239 |
| Hbenzene | 0.239 |
| N | -1.102 |
| B | 0.747 |
| HN | 0.432 |
| HB | -0.077 |
It can be seen from the relative charge distribution that neither benzene nor borazine have a dipole, as a molecule, since the charges are spread evenly across each molecule. Benzene has much more regular electron distribution than borazine, as shown by the much smaller magnitudes of charge on each atom. This is because carbon and hydrogen have very similar electronegativities (2.55 and 2.20, respectively).[3] Since boron and nitrogen have far more different electronegativities (2.04 and 3.04, respectively),[3] the electrons are much more attracted to nitrogen, giving it a partial negative charge, and giving boron a partial positive charge. The hydrogen atoms seem to be primarily affected by the atom to which they're bonded, with the hydrogen atoms bonded to nitrogen showing a partial positive charge (since nitrogen is more electronegative than hydrogen), and the hydrogen atoms bonded to boron showing a very slight partial negative charge (since hydrogen is slightly more electronegative than boron).
Smf115 (talk) 00:33, 27 May 2018 (BST)Same colour range used across both molecules to highlight the charge distribution and good consideration of electronegativities. However, the analysis could ahve been developed further with consideration of symmetry, net neutrality or comparison of bond polarities for example.
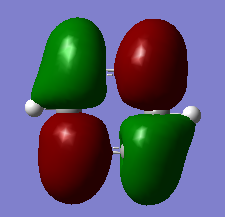
Molecular Orbital Analysis
Smf115 (talk) 00:41, 27 May 2018 (BST)Nice comparison of the MOs chosen and the correct pairs identified by shape and not by energy. To improve, the sigma- or pi- type nature of the MOs could have been identified and other aspects, such as electronegativity or the contiruting MOs, could hav ebeen considered.
Discussion
Historically, many different criteria have been suggested to define aromaticity.[4] Three of them are listed below:
- Aromatic molecules are often more stable than expected for a molecule of that structure. This difference in energy is referred to as the aromatic stabilisation energy,[4] and is a characteristic of all aromatic systems. This energy can be calculated using different models of varying reliability.
- Aromatic molecules are often drawn as having alternating single and double bonds, however, in reality the bond lengths are somewhere between what would be expected for single and double bonds of the same atoms.[4]
- When analysed by proton NMR, aromatic hydrogens appear at a higher chemical shift than what might be expected. This is because the magnetic force produces a ring current in the π-system, which causes any atom just outside the ring to experience a stronger magnetic field, and so are more deshielded.[4]
Simply, aromaticity can be described as a ring system in which each atom of the ring donates electrons from a pz orbital to a π system above and below the plane of the molecule. However this is a very simplistic approach, and in some cases does not allow one to accurately identify an aromatic system. The varying criteria of aromaticity can different results depending on which criteria are used to define aromaticity,[4] and so this definition is very restrictive.
The aromatic MOs calculated during this project consist of three occupied and three unoccupied orbitals. There is one relatively low energy orbital shown in the Molecular Orbital Analysis section which shows an electron cloud above and below the molecule, with a σh nodal plane, due to the contributing pz-orbitals. The other occupied aromatic orbitals are very similar, but also have a σv nodal plane, one along the x axis, and one along the y axis. These two are degenerate. These orbitals represent an electron cloud, as described by common conceptions of aromaticity.
Smf115 (talk) 00:43, 27 May 2018 (BST)Overall a well presented wiki with a particularly good first section.
References
- ↑ P. Hunt, Molecular Orbitals Lecture Course, Pippard Lecture Theatre, Imperial College London, November, 2017.
- ↑ Wired Chemist, http://www.wiredchemist.com/chemistry/data/bond_energies_lengths.html, accessed 16th May 2018
- ↑ 3.0 3.1 Science Notes, https://sciencenotes.org/list-of-electronegativity-values-of-the-elements/, accessed 17th May 2018
- ↑ 4.0 4.1 4.2 4.3 4.4 M. Palusiak and T. M. Krygowski, Chem. - Eur. J., 2007, 13, 7996-8006, DOI:10.1002/chem.200700250