I07t:Zithromycin
Zithromycin
zithromycin |
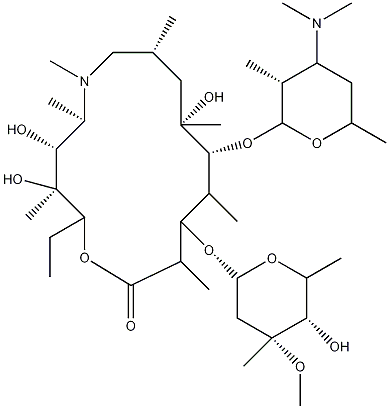
| IUPAC NAME | (2R,3R,4R,5R,8R,10R,11R,13S,14R)-11-[(2S,3R,4S,6R)-4-dimethylamino-3-hydroxy-
6-methyl-oxan-2-yl]oxy-2-ethyl-3,4,10-trihydroxy-13-[(2S,4R,5S,6S)-5-hydroxy-4-methoxy-4,6-dimethyl- oxan-2-yl]oxy-3,5,6,8,10,12,14-heptamethyl-1-oxa-6-azacyclopentadecan-15-one dihydrate |
|---|---|
| CAS number | 83905-01-5 |
| ATC code | J01FA10 |
| PubChem | 55185 |
| Chemical formula | C38H72N2O12 |
| Molar mass | 748.88 |
| half life | 68hours |
| metabolism | hepatic |
| melting point | 113-115°C |
| smiles strings | CCC1C(C(C(N(CC(CC(C(C(C(C(C(=O)O1)C)OC2CC(C(C(O2)C)O)(C)OC)C)OC3C
(C(CC(O3)C)N(C)C)O)(C)O)C)C)C)O)(C)O |
| Pregnancy risk | B1(Aus) |
Synonyms
Azithromycin,azithromycin dihydrate, azithromycin hydrate, zithromax.
General
Zithromycin is a derivative of the antibiotic erythromycin but with a nitrogen atom substituting the methyl group in the lactone ring. This makes it a macrolide antibiotic - having a large macrocylic lactone ring with 14, 15 or 16-carbons.
This partially synthesized macrolide antibiotic is chemically related to erythromycin and clarithromycin (biaxin). It is orally administered and is used in the treatments of infections in the lower and upper respiratory tracts and skin and soft tissues.
(1)Patients with weakened immune systems, including people with HIV, tend to have more frequent and more serious bacterial infections. Zithromycin was approved by the FDA on June 14, 1996, for many uses, including the prevention and treatment of Mycobacterium avium complex (MAC) in persons with advanced HIV infection. It is also being investigated to see how well it works in preventing other kinds of bacterial infections in people with HIV.
Microbiology
The meachanism of action for zithromycin is similar to its prototype, erythromycin. It interferes with the bacteria's protein synthesis by binding to the 50S component of the 70s ribosomal subunit.
Zithromycin is slightly less potent with gram-positive bacteria compared to erythromycin, but is best against a large variety of gram-negative microorganisms. Some examples of gram-negative microorganisms are Staphylococcus aureus, Staphylococcus pneumoniae, Staphylococcus pyogenes, Haemophilus influenzae and Moraxella catarrhalis.
Zithromycin also actively reacts against other pathogens such as Borrelia burgdorferi, Toxoplasma gondii, Mycobacterium avium complex, and Mycobacterium leprae. This antibiotic is also used in treating uncomplicated urethritis/cervicitis associated with sexually transmitted infections Neisseria gonorrhoeae, Ureaplasma urealyticum and especially, Chlamydia trachomatis.
However care must be taken in the treatment of urethritis and cervicitis, as the use of zithromycin may mask the symptoms of incubating gonorrhea or syphilis. Doctors must take care to take a full background on the illness, and cause of, before confirming treatment with zithromycin.
Side effects
Common side-effects of zithromycin are nausea, vomitting, abdominal pain and diarrhoea. These gastrointestinal side-effects are less prevalent in the use of zithromycin when compared to its prototype, erythromycin. Zithromycin can be safely used during pregnancy and breast-feeding.
Metabolism
Its metabolism is largely hepatic and is mainly eliminated from the body via biliary excretion. This elimination is biphasic, having a terminal half-life of up to 5 days.
Pharmacokinetics
Approximately 37% is absorped after taking a 500-mg oral dose of zithromycin. This antibiotic demonstrates a large volume distribution of 23L/kg and a low peak serum level of 0.4 μg/ml, affirming its extensive tissue ditribution and intracellular accumulation. This results in zithromycin having a long tissue half-live of about 3 days. Hence, allowing once-daily or twice-daily dosage and shorter treatment term for most infections.
Bibliography
http://www.chemindustry.com/apps/chemicals?m=s&t=AZITHROMYCIN
http://www.patient.co.uk/showdoc/30002446/
http://www.medicinenet.com/azithromycin/article.htm
http://aidsinfo.nih.gov/DrugsNew/DrugDetailNT.aspx?MenuItem=Drugs&Search=On&int_id=104 (for reference(1))
http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=2809 (for the structure)
