Hg3117 summer 1
Exercise 1: H + H2 System
***On a potential energy surface diagram, how is the transition state mathematically defined? How can the transition state be identified, and how can it be distinguished from a local minimum of the potential energy surface?***
The transition state can be mathematically defined as the point where the gradient=0 (d(V(ri))/d(ri)=0). We can identify the transition state as where the maximum on the minimum energy pathway linking the products and reactants forms a saddle point with the minima on the potential energy surface.
A bit confusing, but I think that's due to infelicitous wording. Consider a more mathematical language here, e.g. '... as where the minimum energy pathway connecting two minima on the potential energy surface reaches a maximum, which is characterized as a first-order saddle point.' Fdp18 (talk) 09:50, 4 May 2019 (BST)
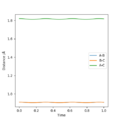
***Report your best estimate of the transition state position (rts) and explain your reasoning illustrating it with a “Internuclear Distances vs Time” plot for a relevant trajectory.***
The transition state position of the reaction was found by setting both internuclear distance to be the same and then altering them by trial and error. Both momenta values were set to 0 so that all particles were stationary.
The estimated r(ts) for this reaction was 0.91. The reasoning for this value is it is the value in which there was the least amount of symmetric oscillation and the reaction coordinate was most centred on the minimum energy pathway maximum. An 'Internuclear Distance vs Time' plot showing the relevant atom trajectories has been uploaded below.
***Comment on how the mep and the trajectory you just calculated differ.***
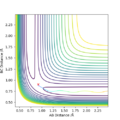
An minimum energy pathway (mep) trajectory was set up. The mep trajectory resets the particles momenta and velocity to 0 at every step and so the trajectory corresponds to infinitely slow motion.
The values put intot he calculation were r1 = rts +0.01 and r2 = rts with both momenta being kept at 0.
The results showed that a bond formed between the two closer particles (B and C) and internuclear distance began oscillating as in previous calculations as the bond vibrated and the distance AB increased as the A particle moved away. When the values were reversed and A and B were initially closer the same results were observed except the particles roles were reversed.
The mep and dynamic trajectories differed in that the dynamic trajectory took into account the vibrational motion of the bond within the H2 molcule and the mep trajectory didn't.
The difference can be seen in the two images uploaded below. In the dynamic type calculation, the trajectory can clearly be seen to oscillate slightly.
In the initial calculation the final internuclear distance of AB and BC were found as 3.45 and 0.725 respectively and the average momenta at large t were seen as 2.47 and 1.24 respectively. These values were put into a calculation and in the results it was seen that the AB distance decreased as the BC molecule and A particle came closer then a minimum AB internuclear distance was reached. The AB distance was still larger than the BC distance so the BC bond was retained and then the AB particle increased at the same rate that it initially decreased. This can be seen on the figure below:
***Complete the table above by adding the total energy, whether the trajectory is reactive or unreactive, and provide a plot of the trajectory and a small description for what happens along the trajectory. What can you conclude from the table?***
Trajectories were run over various initial momenta with constant initial internuclear distances of r1 = 0.74 and r2 = 2.0 angstroms. This was done to see if even though kinetic energy was greater than the smallest kinetic energy required to initiate the reaction, the reaction would still occur.
| p1 | p2 | Etot/J | Reactive? | Description of the dynamics |
|---|---|---|---|---|
| -1.25 | -2.5 | -9.9 | Y | AB oscillates and BC gets smaller, reach rts and reaction occurs BC oscillates and AB increased. |
| -1.5 | -2.0 | -10.0 | N | BC distance decreases but doesn't reach rts as momentum likely too low. BC then increases again as reaction doesn't occur. |
| -1.5 | -2.5 | -9.9 | Y | Reaction occurs as normal |
| -2.5 | -5.0 | -8.5 | N | AB oscillation very slightly on approach and BC distance decreases rapidly. Once the rts is reached there is a single oscillation between B and C and A is kept from moving away much. At maximum separation of the BC oscillation the bond between A and B reforms and C then moves away with no overall reaction occurring. |
| -2.5 | -5.2 | -8.34 | Y | Reaction initially starts the same as previous example but the AB distance increases too much after initial collision so that the bond isn't reformed and the reaction goes ahead. |
Reconsider your Energies and take a look at the units. An energy of 1 J corresponds to ~100000000000000000000 kJ/mol, which is a lot. Fdp18 (talk) 10:01, 4 May 2019 (BST)
Where the conditions are p1 = -2.5 and p2 = -5.0 something called barrier recrossing is observed.
***State what are the main assumptions of Transition State Theory. Given the results you have obtained, how will Transition State Theory predictions for reaction rate values compare with experimental values?***
The main assumptions made in Transition State Theory are firstly that the energy distribution are Boltzmann Distributed and that this can be satisfied so long enough time has passed so that thermal equilibration has been reached. It is also assumed that the reactants are all thermally equilibrated. Another assumption is that once the transition state has been reached then the reaction cannot re-cross the barrier and revert back into the reactant state.
I would recommend you to present such assumptions in a list, which makes it easier to follow. Additionally, be careful with your wording, 'the energy distribution are Boltzmann Distributed' sounds a bit vague. Consider something like 'It is assumed that the distribution of the particles to their possible states follows Boltzmann statistics'.Fdp18 (talk) 10:13, 4 May 2019 (BST)
Experimentally the reaction rate would be lower than the one predicted by Transition State Theory as there are more reaction pathways accessible as seen in the penultimate result from the above table where there is barrier recrossing. This means that not all reactions that have the correct energy will go to completion so rate will be reduced.
Exercise 2: F - H- H System
***By inspecting the potential energy surfaces, classify the F + H2 and H + HF reactions according to their energetics (endothermic or exothermic). How does this relate to the bond strength of the chemical species involved?***
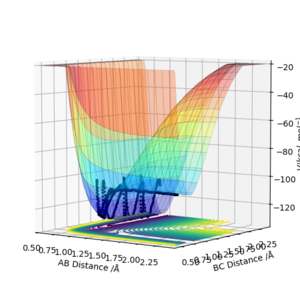
By looking at the energy potential curves of the F + H2 and H + HF reactions it was determined that they were exothermic and endothermic respectively. The potential energy surface of the F + H2 reaction is shown below, to set up this calculation the AB distance was set at 2.3 angstroms and the BC distance set at 0.74 angstroms to ensure the initial bond was between the two H atoms. P1 was set as -2 and p2 was set as -1.5. The HF bond is much stronger than the HH bond, so overall energy will be released into the surroundings, as more energy released than taken in, making the reaction exothermic. In the surface plot it can be seen that there is an overall decrease in potential energy as the HF bond is formed.
The potential energy surface of the HF + H reaction is shown below. The values used for this calculation were 1.21 and 2.24 angstroms respectively for AB and BC, and 1.5 and -1.5 respectively for p1 and p2 . This reaction was endothermic as the HF bond is much stronger than the HH bond so overall the energy needed to break the HF bond is greater than the energy released when the HH bond formed, meaning overall there was energy put into the reaction.
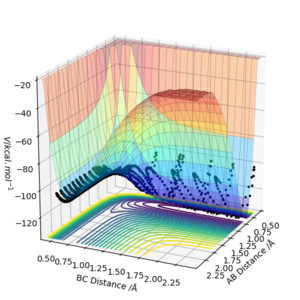
***Locate the approximate position of the transition state***
Unlike the previous H + H2 example, the transition state won't be in position where H and F are equidistant from the middle H due to F being larger. The transition state position is found approximately at AB = 1.6 angstroms and BC = 0.75 angstroms. Both momentums were set to 0. Due to one of the reactions having a small activation energy Hammond's postulate was used to help find the correct position. The Hammond Postulate states that the transition state is close in structure to the side of the reaction that it has the most similar energy too. The more endothermic a reaction is, the closer to the product's structure it is, the exothermic, the closer to the reactants structure it is.
***Report the activation energy for both reactions***
The activation energy for both reactions was found by using a structure very similar to the transition state and using an mep trajectory. r1 = r1ts - 0.1 and r2 = r2ts. The activation for the reaction H2 + F has 0 activation energy as minimum energy pathway just decreases with no maximum, the reaction of HF + H has an activation energy of 29.0 kcal/mol. This value was found by looking at the initial and final potential energy of the transition state with the products.
Think about the consequences of a reaction with no barrier. Would it be sensible in this case? Some random point at the 'Wall' of the PES also relaxes to a minimum without barrier - would this be a sensible 'starting material'? Then, try to imaging the Energy as a function of position, i.e. as a pass in a long valley. If you found an actual activation energy for one side - the 29 kcal/mol - then there has to be one for the other side, too, even if it is very small. As you correctly write, the 'minimum energy pathway just decreases' - provided that your TS guess was valid, than you already know that there has to be a barrier. You just have to choose your initial conditions accordingly - imagine placing a sphere on the TS position, tilting it a bit in the two directions so it rolls downhill. Fdp18 (talk) 10:36, 4 May 2019 (BST)
The part with the release of reaction energy is missing, as well as thoughts about experiments. Consider how the difference in potential energy is converted into other forms of energy, since the total energy has to be constant. Think back to the gaussian labs - what experimental technique do you use to investigate the vibrations (which are directly connected to the second derivatives)?Fdp18 (talk) 10:36, 4 May 2019 (BST)
The last question about the efficiency of the reaction is also missing. The keyword to look for here is 'Polanyi's rules'...Fdp18 (talk) 10:42, 4 May 2019 (BST)
Last, but definitively not least important: Please include all references, like the ones where you found the assumptions of transition state theory.Fdp18 (talk) 10:14, 4 May 2019 (BST)
Overall, the report is very minimal, and some parts are missing completely. I assume time management was the issue at the end? Please also consider structuring your reports appropriately with different sections. --> 2 MarksFdp18 (talk) 10:45, 4 May 2019 (BST)