HariChauhanInorganic
BH3
B3LYP/6-31G Level
Item Value Threshold Converged? Maximum Force 0.000014 0.000450 YES RMS Force 0.000007 0.000300 YES Maximum Displacement 0.000053 0.001800 YES RMS Displacement 0.000027 0.001200 YES
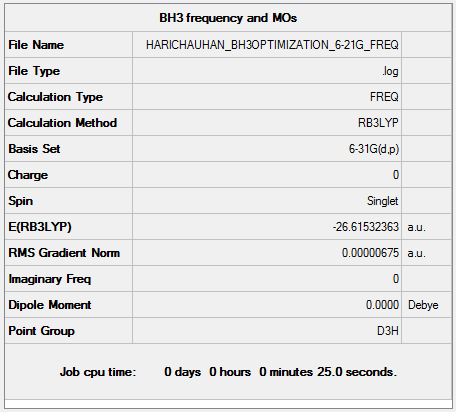
Low frequencies --- -7.5936 -1.5614 -0.0055 0.6514 6.9319 7.1055 Low frequencies --- 1162.9677 1213.1634 1213.1661
BH3 |
Vibrational spectrum for BH3
| wavenumber (cm-1) | Intensity (arbitrary units) | symmetry | IR active? | type |
| 1163 | 93 | A2" | yes | out-of-plane bend |
| 1213 | 14 | E' | slightly | bend |
| 1213 | 14 | E' | slightly | bend |
| 2582 | 0 | A1' | no | symmetric stretch |
| 2716 | 126 | E' | yes | asymmetric stretch |
| 2716 | 126 | E' | yes | asymmetric stretch |
Only three peaks appear in the predicted spectrum because there are two pairs of E' vibrational modes that are degenerate, hence they appear as one signal each. Furthermore, the A1' symmetric stretch does not produce a change in the dipole moment of the molecule so is not IR active.

The a1' molecular orbital looks different from that predicted by LCAO. The s orbitals on the hydrogen atoms appear much larger in the calculated MO. This suggests that the LCAO approach to predicting molecular orbitals may have some inaccuracies.
Ng611 (talk) 19:48, 4 June 2018 (BST) I'd say the a1' MO looks pretty much like that predicted from qualitative MO theory. Are there any particular MOs in which the hydrogen s-orbitals are larger than predicted? It's important to remember that overall, differences between these real MOs and the computed ones?
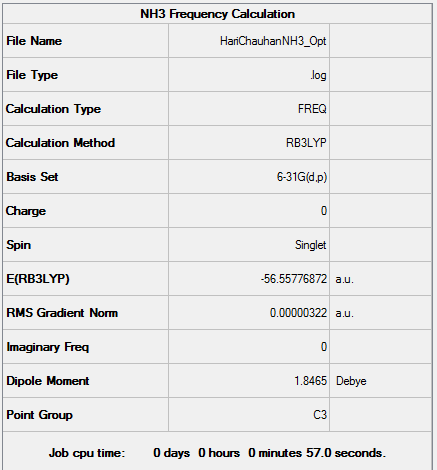
NH3
B3LYP/6-31G Level
Item Value Threshold Converged? Maximum Force 0.000006 0.000450 YES RMS Force 0.000003 0.000300 YES Maximum Displacement 0.000013 0.001800 YES RMS Displacement 0.000007 0.001200 YES
Low frequencies --- -0.0138 -0.0032 -0.0015 7.0783 8.0932 8.0937 Low frequencies --- 1089.3840 1693.9368 1693.9368
NH3 |
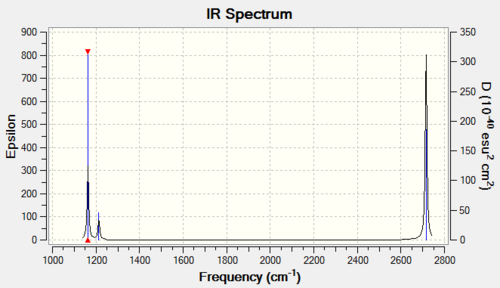
Vibrational spectrum for NH3
| wavenumber (cm-1) | Intensity (arbitrary units) | symmetry | IR active? | type |
| 1089 | 145 | A | yes | asymmetric strech |
| 1693 | 14 | E | slightly | bend |
| 1693 | 14 | E | slightly | bend |
| 3461 | 1 | A1' | very slightly | symmetric stretch |
| 3589 | 0 | E | no | asymmetric stretch |
| 3589 | 0 | E | no | asymmetric stretch |
Similar to BH3, NH3 has two sets of degenerate vibrational modes. However one of these pairs is IR inactive. Therefore, only three signals are predicted in the IR spectrum.
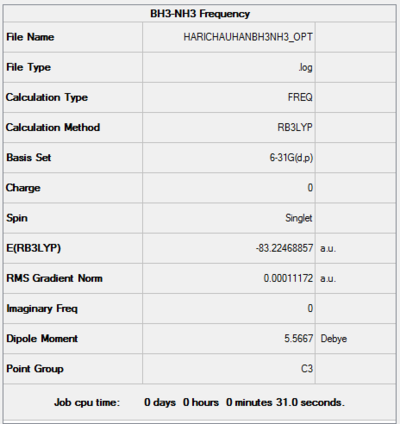
BH3NH3
B3LYP/6-31G Level
Item Value Threshold Converged? Maximum Force 0.000350 0.000450 YES RMS Force 0.000112 0.000300 YES Maximum Displacement 0.001350 0.001800 YES RMS Displacement 0.000450 0.001200 YES
Low frequencies --- -0.0262 -0.0082 -0.0023 9.6688 9.6769 37.9651 Low frequencies --- 265.3241 634.4274 639.1700
NH3 |
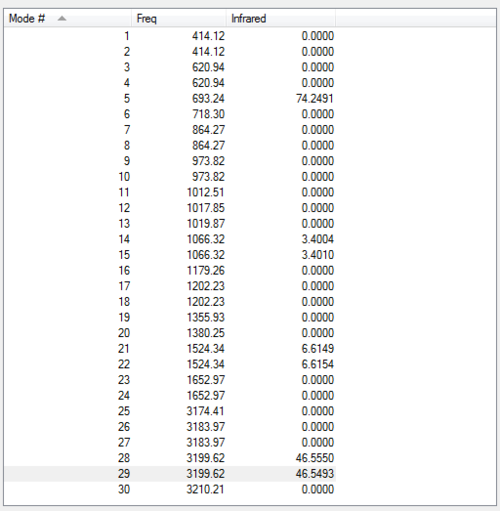
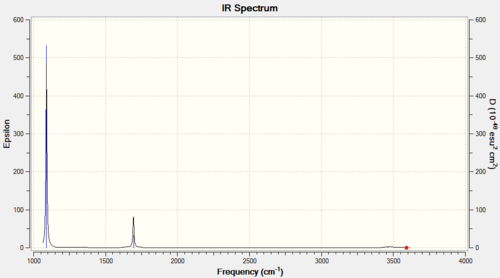
Vibrational spectrum for BH3NH3
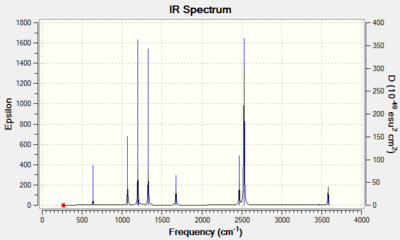
Due to the large number of vibrational modes in BH3NH3, a screenshot of the vibrations predicted by Gaussian is provided. A total of 11 bands should be observed, although some with very low intensity. Also, some are very close in energy so may overlap.
Calculating B-N Bond Energy in BH3NH3
E(NH3)= -56.55777 a.u. E(BH3)= -26.61532 a.u. E(NH3BH3)= -83.22469 a.u. ΔE=E(NH3BH3)-[E(NH3)+E(BH3)] = -83.22469 - (-56.55777 - 26.61532) = -0.05167 a.u. -0.05167 a.u. * 2625 = -136 kJmol-1
The energy of the B-N bond in BH3NH3 is -136 kJmol-1
Ng611 (talk) 19:52, 4 June 2018 (BST) You're off by 1 Kj/mol in your final answer (copy/pasting your working into google calculator gives the right answer, so it looks like you might have mistyped a digit). Remember also to cite your bond values (ideally from a textbook, databook, or paper).
Comparing this bond with the C-C bond strength in ethane, which is isoelectronic, the B-N bond is significantly weaker. In ethane, the C-C bond is around 364 kJmol-1, over double that of the B-N bond strength. This is likely due to the large electronegativity difference between B and N.
BBr3
B3LYP/6-31G(d,p)LANL2DZ Level
Item Value Threshold Converged? Maximum Force 0.000010 0.000450 YES RMS Force 0.000005 0.000300 YES Maximum Displacement 0.000045 0.001800 YES RMS Displacement 0.000022 0.001200 YES
Low frequencies --- -0.0135 -0.0064 -0.0046 2.4669 2.4670 4.8600 Low frequencies --- 155.9626 155.9646 267.7022
BBr3 |
Vibrational spectrum for BBr3
| wavenumber (cm-1) | Intensity (arbitrary units) | symmetry | IR active? | type |
| 156 | 0 | E' | no | bend |
| 156 | 0 | E' | no | bend |
| 268 | 0 | A1' | no | symmetric stretch |
| 378 | 4 | A2" | slightly | out-of-plane bend |
| 763 | 320 | E' | yes | asymmetric stretch |
| 763 | 320 | E' | yes | asymmetric stretch |
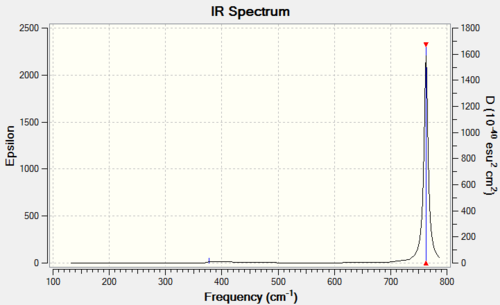
Only one peak is seen in this spectrum as the two stretches at 763 cm-1 have far higher intensity than the other IR active stretch at 378 cm-1.
Benzene
B3LYP/6-31G Level
Item Value Threshold Converged? Maximum Force 0.000194 0.000450 YES RMS Force 0.000084 0.000300 YES Maximum Displacement 0.000767 0.001800 YES RMS Displacement 0.000328 0.001200 YES
Low frequencies --- -16.9682 -14.6636 -14.6636 -0.0054 -0.0054 0.0004 Low frequencies --- 414.1239 414.1239 620.9400
Benzene |
Vibrational spectrum for Benzene
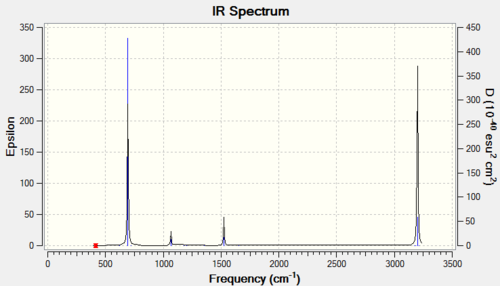
Although benzene clearly has very many vibrational modes, due to its high symmetry, only a few are IR active. This explains why only 4 peaks are observed in its IR spectrum.
Borazine
B3LYP/6-31G Level
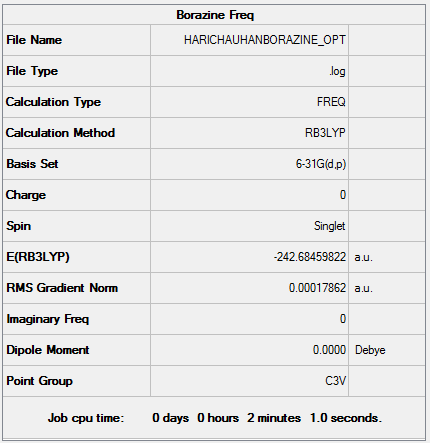
Item Value Threshold Converged? Maximum Force 0.000443 0.000450 YES RMS Force 0.000179 0.000300 YES Maximum Displacement 0.000882 0.001800 YES RMS Displacement 0.000400 0.001200 YES
Low frequencies --- -0.0045 -0.0033 -0.0031 5.3417 5.3626 5.9709 Low frequencies --- 289.8780 289.8786 404.4105
Borazine |
Vibrational spectrum for Borazine
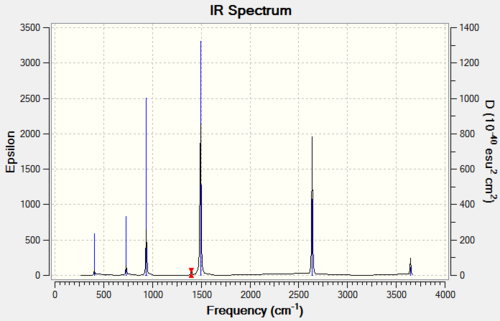
Borazine has the same number of vibrational modes as benzene, however, due to its lower symmetry, more of these are IR active, which is why it shows 6 active signals in the IR spectrum.
Comparison of Benzene and Borazine
Comparison of Charge
| Benzene | Borazine |

|

|
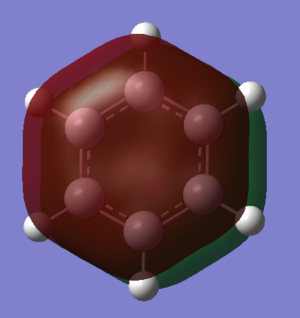
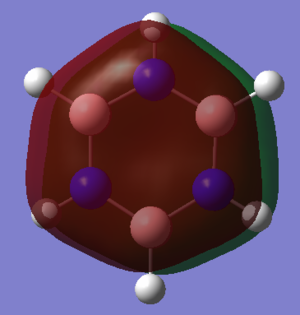
The brighter colours imply a higher magnitude of charge, with red implying a negative charge and green implying a positive charge.
Because carbon and hydrogen have very close electronegativities, the C-H bond has a large amount of covalent character. The C-C bond, being homonuclear, is completely covalent. Therefore, there is little separation of charge in benzene. Because carbon is a little more electronegative than hydrogen, a negative charge of -0.239 resides on the carbon atoms, with +0.239 on the hydrogen atoms. However, due to the D6h point group of the molecule, the sum of the C-H bond dipoles is zero, giving the benzene molecule an overall zero dipole moment.
In borazine, there is a higher separation of charge, shown by the brighter colours in the figure above. Nitrogen has a significantly higher electronegativity than boron and hydrogen, making the N-B and N-H bonds have a large dipole moment. Comparing the B-H and N-H bonds, the hydrogen atoms bonded to boron show little charge but the hydrogen atoms bonded to nitrogen have a charge of +0.432 as electrons in the N-H bond are drawn closer to the nitrogen.
In borazine, a large amount of negative charge resides on the Nitrogen atoms, with the boron and hydrogen atoms having positive charge, however due to the D3h point group of the molecule, the dipole moments of these polar bonds sums to zero, to give borazine no overall dipole moment.
Both molecules have a total charge of zero, so summing up the total charge gives zero in both cases.
Comparison of Molecular Orbitals
Aromaticity
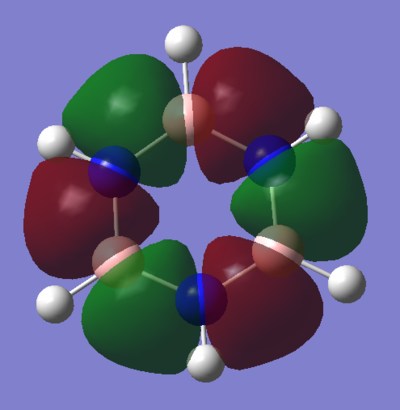
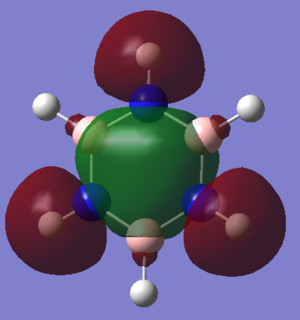
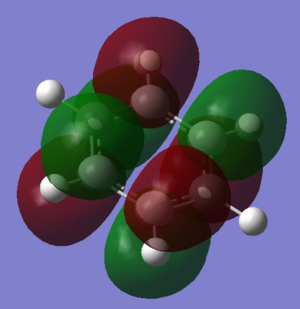
Hückel proposed a few criteria which a molecule must meet in order to be considered aromatic. It must be cyclic, planar, have a continuous system of p-orbitals adjacent to the ring and contain 4n+2 π-electrons in this π-system. This approach to aromaticity suggests that the aromatic stabilization of benzene comes largely from the molecular orbital shown above with an area of electron density above and below the ring of the molecule (MO 17).
However, it is now well known that planarity is not a requirement for aromaticity. At 20K, it has been shown that benzene, in a crystal structure, adopts a chair conformation.[1] This shows a flaw in Huckel's theory and suggests that there is much more to aromaticity that he originally suggested. In fact, aromaticity is a word used to describe a number of stabilizing effects with the planar π-system being only one of these.
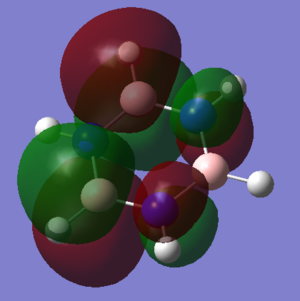
Sigma aromaticity, for example, is displayed in the MO shown. This is caused by the in-phase overlap of p-orbitals in the plane of the ring that face along the B-N bonds. This allows electrons in these p-orbitals to also delocalise over the aromatic molecule. Sigma aromaticity also exists in molecules that don't resemble anything suggested by Huckel. For example, stabilization due to aromaticity and destabilization due to anti-aromaticity has been shown to exist in inorganic cages.
Ng611 (talk) 19:57, 4 June 2018 (BST) Some really good points made here. I would expand on this discussion somewhat, with more reference to more recent models of aromaticity, and perhaps a discussion on experimental results that demonstrate aromaticity in these molecules?