HMR17 IMM2
Appearance
NH3 Optimization
| Calculation Type | FREQ (opt-freq) | Optimized N-H Bond Length | 1.01798 Å |
| Basis Set | 6-31G(d.p) | Optimized H-N-H Angle | 105.74115° |
| E(RB3LYP) | -56.55776873 a.u. | Charge on Nitrogen | -1.125 |
| RMS Gradient | 0.00000485 a.u. | Charge on Hydrogen | 0.375 |
| Point Group | C3v |
The negative charge on nitrogen matches expectations based on its high electronegativity compared to hydrogen, with the corresponding positive charge spread equally over the hydrogen atoms.
Item Value Threshold Converged? Maximum Force 0.000004 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000072 0.001800 YES RMS Displacement 0.000035 0.001200 YES
NH3 |
File:HRICKARD NH3 OPTF POP.LOG
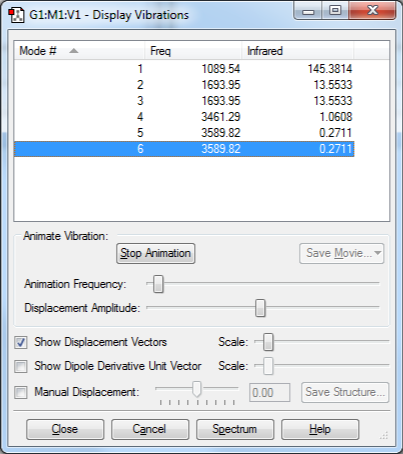
- Expected number of vibrational modes from 3N-6 rule is 6.
- Modes 2 and 3, and 5 and 6 are degenerate.
- Modes 1, 2, and 3 are "bending vibrations", and 4, 5, and 6 are "stretching vibrations".
- Mode 4 is highly symmetric.
- Mode 1 is (probably) known as the "umbrella" mode.
- In gaseous-phase ammonia, 3 bands would be seen in the IR spectrum under ideal conditions (very high signal-to-noise ratio). In practice, the band for modes 5 and 6 would likely be so small as to be invisible compared to those for 1 and 2/3.
N2 Optimization
| Calculation Type | FREQ (opt-freq) |
| Basis Set | 6-31G(d.p) |
| E(RB3LYP) | -109.52412868 a.u. |
| RMS Gradient | 0.00000365 a.u. |
| Point Group | D∞h |
Item Value Threshold Converged? Maximum Force 0.000006 0.000450 YES RMS Force 0.000006 0.000300 YES Maximum Displacement 0.000002 0.001800 YES RMS Displacement 0.000003 0.001200 YES
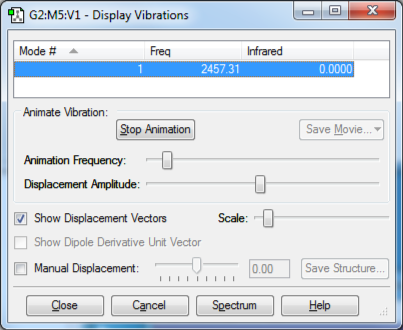
The only vibrational mode of N2 is its stretch.
N2 |
H2 Optimization
| Calculation Type | FREQ (opt-freq) |
| Basis Set | 6-31G(d.p) |
| E(RB3LYP) | -1.17853935 a.u. |
| RMS Gradient | 0.00003809 a.u. |
| Point Group | D∞h |
Item Value Threshold Converged? Maximum Force 0.000066 0.000450 YES RMS Force 0.000066 0.000300 YES Maximum Displacement 0.000087 0.001800 YES RMS Displacement 0.000123 0.001200 YES
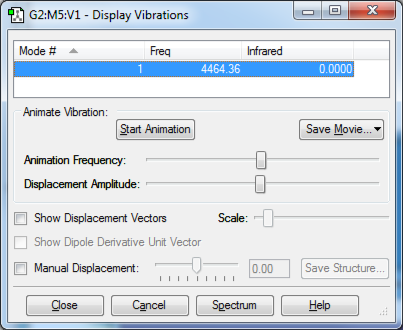
The only vibrational mode of H2 is its stretch, similarly to N2.
H2 |
Haber-Bosch Process Calculations
3H2 + N2 → 2NH3
E(NH3) = -56.55776873 a.u.
2*E(NH3) = -133.1155375 a.u.
E(N2) = -109.5241287 a.u.
E(H2) = -1.17853935 a.u.
3*E(H2) = -3.53561805 a.u.
ΔE = E(products) - E(reactants) = -0.05579073 a.u. = -146.48 kJ mol-1
The negative energy change shows that the reaction is exothermic and the NH3 product is lower in energy (i.e. more stable) than the reactants.
H2SiO Optimization
| Calculation Type | FREQ (opt-freq) | Charge on Oxygen | -1.001 |
| Basis Set | 6-31G(d.p) | Charge on Silicon | 1.472 |
| E(RB3LYP) | -365.90001403 a.u. | Charge on Hydrogen | -0.236 |
| RMS Gradient | 0.00000941 a.u. | Optimized O-Si-H Angle | 124.15648° |
| Point Group | CS | Optimized H-Si-H Angle | 111.68596° |
| Dipole Moment | 3.4340 D |
H2SiO |
File:HRICKARD H2SIO OPTF POP.LOG
Item Value Threshold Converged? Maximum Force 0.000023 0.000450 YES RMS Force 0.000009 0.000300 YES Maximum Displacement 0.000023 0.001800 YES RMS Displacement 0.000017 0.001200 YES