Grt07:breakyourself
Module 1
Hydrogenation of Cyclopentadiene Dimer
 |
 |
 |

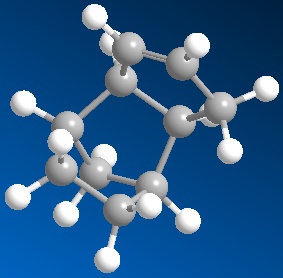 |
Cyclopentadiene[1] dimerises at room temperature via a Diels-Alder mechanism to give dicyclopentadiene(right). The endo-dimer is produced exclusively over the exo-dimer. The reasons for this were investigated by minimising the energy of the conformations using the Allinger MM2 molecular mechanics model feature in ChemBio3D Ultra and comparing the results(Table 1).
The total energies of the exo and endo dimers were 133.49 and 142.38 KJmol-1 respectively. This suggests that, thermodynamically, the exo product is more stable. Since we know that the less thermodynamically stable endo dimer is produced exclusively over the exo equivalent we can assume that the reaction is kinetically controlled. This is indeed the case as the dimerisation reaction proceeds via an irreversible diels alder reaction and if the reaction was not irreversible, eventually the thermodynamically more stable endo product would dominate.
The largest contribution to the total energy of both molecules is the bend energy. This probably arises from the interactions of the bridgehead with the adjacent hydrogens which causes steric clashing and hence an increase in the total energy of the molecule. In the exo-product the 2 adjacent hydrogens on the opposite side of the molecule which slightly reduces the energy.
The main difference in the total energy of the 2 dimers is the torsion energy. The endo-dimer has a higher torsion energy which, by examining the 3D structures of the 2 dimers (Jmols, right) it is clear that the endo-dimer is much more twisted than the exo equivalent resulting in the increased torsion energy of the endo-dimer.
The endo dimer can be hydrogenated to form the dihydro product 1 or 2. Using the MM2 molecular mechanics model, the energies of the two possible products were calculated and the results shown in Table 1. We can see that Product 1 and 2 have total energies of 146.41 and 130.48 KJmol-1 respectively and hence it can be concluded that Product 2 is thermodynamically more stable. The main contributor to the difference in total energy between the two products is the bend energy. Dihydro product 1 has a bend energy of 79.729 KJ-1 compared to dihydro product 2 which has a bend energy of 60.738 KJ-1 giving a lower bend energy of 18.991 KJ-1 for dihydro isomer 2. The bend energy is associated with the compressing and stretching of bond angles within a molecule. This large difference in energy, in this case, must be due to the location of the double bond. Double bonds are shorter than single bonds between the same atoms and if we consider that the double bond that is hydrogenated to a single bond (lengthening the bond) in dihydro product 2 probably reduces some strain on the bridging carbon bond angles shown as pointing down in the chemdraw diagram (right).
| Energy Type | Exo Dimer | Endo Dimer | Dihydro Product 1 | Dihydro Product 2 |
|---|---|---|---|---|
| Stretch | 5.4106 | 5.2461 | 5.2151 | 4.6239 |
| Bend | 86.194 | 87.316 | 79.729 | 60.738 |
| Stretch-Bend | -3.5224 | -3.4851 | -3.4654 | -2.3006 |
| Torsion | 32.119 | 39.782 | 46.783 | 52.378 |
| Non-1,4-VdW | -6.0114 | -6.3602 | -6.7826 | -4.4137 |
| 1,4-VdW | 17.719 | 18.02 | 24.255 | 18.859 |
| Dipole-Dipole | 1.5818 | 1.8652 | 0.67952 | 0.58866 |
| Total | 133.49 | 142.38 | 146.41 | 130.48 |
Stereochemistry of Nucleophilic additions to a pyridinium ring (NAD+ analogue)
Reactant molecules 1 and 2 were optimized using MM2 and MMFF94 molecular mechanics methods. The molecules were then altered and reoptimized in an attempt to lower the total energies with the final results shown below (Table 2). The orientation of the carbonyl group itself and the neighbouring groups which may interact with the carbonyl were the main focus of the alterations.
| Molecule | MM2 | MMFF94 |
|---|---|---|
| Molecule 1 | 179.26 | 240.6 |
| Molecule 2 | 284.81 | 411.54 |

 |
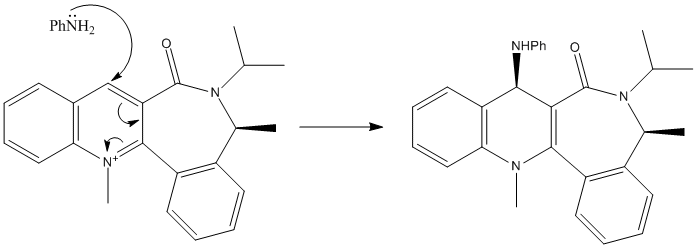
For reactant molecule 1, it seems that the lowest energy conformation is when the carbonyl oxygen is equatorial to the 7 membered ring which minimises the effects of the 1,3- and 1,4- diaxial effects that would act to increase the overall energy of the molecule. We can see how the product is formed if we consider the mechanism (left). The electropositive magnesium from the grignard reaction interacts with the electronegative carbonyl oxygen, 'holding' the MeMgI molecule close to the 4-carbon in the pyridinium ring, allowing the negatively charged methyl group to attack it in the 4-position.
ChemBio3D is not programmed to recognise metal atoms and the fact that the grignard reagent does not form a complete sigma or pi bond with the reactant means that any calculations that ChemBio3D attempts to make using the MM2 or MMFF94 forcefield leads to misleading results.
For reactant molecule 2, the lowest energy conformation seemed to be when the carbonyl oxygen was pointing slightly down from the ring, away from the propyl group on the nitrogen and the methyl group in the 3 position. In turn, this conformation allows for the attack on the carbon in the 4-position on the pyridial ring, from above, with little steric hinderance, leading to the conformation seen in the product. This is shown in the diagram of the mechanism (left).
Stereochemistry and Reactivity of an Intermediate in the Synthesis of Taxol
| Intermediate | MM2 | MMFF94 |
|---|---|---|
| 1 (up) | 175.21 | 253.88 |
| 2 (down) | 180.47 | 253.69 |
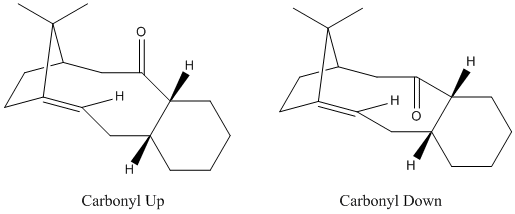
There are 2 possible isomers of an intermediate in the total synthesis of taxol (right). In intermediate 1 the carbonyl group is pointing up and in intermediate 2 the carbonyl group is pointing down. Atropisomerism has been observed in both forms of the isomer. Atropisomers are stereoisomers resulting from hindered rotation about single bonds where the steric strain barrier to rotation is high enough to allow for the isolation of the conformers. On standing the compound isomerises to the alternative carbonyl isomer.
The energies of the 2 isomers were minimised with both the MM2 and MMFF94 force fields and the results shown below (Table 4). The carbonyl up isomer had to be manually altered to get the carbonyl group to remain pointing up when optimized which clearly indicated that this was the less thermodynamically favourable. By studying the 3D models of the molecules it is clear to see where the main contribution to the large difference in the energies of the 2 isomers originates after the initial minimization. With the carbonyl group pointing up there is clearly a lot of strain caused by the interactions of the carbonyl with the bridging isopropyl group. However, in the carbonyl down isomer, the carbonyl is on the other side of the molecule to the bulky bridging isopropyl group which dramatically reduces the strain caused by this steric clash.
Optimization of the isomers using the MMFF94 forcefield rather than MM2 gives higher energy results but in terms of which isomer is more stable, the same result is obtained. After alot of manipulation of the carbonyl up intermediate however, much lower energies were observed (these are the energies displayed in Table 2).
These isomers have been described as hyperstable olefins i.e. the functionalisation of the alkene is abnormally slow. Hyperstable olefins have negative olefin strain energies which means that the alkene is lower in energy than the equivalent saturated hydrocarbon so if the alkene is hydrogenated it results in a higher energy molecule. This is due to the change in hybridisation of the sp2 carbon atoms in the C=C double bond to sp3 to form the C-C single bond. When this occurs in a medium sized cyclic system with a bridgehead double bond this decrease in bond angle increases the tortional strain associated with the molecule and hence increases the total energy.
Regioselective Addition of Dichlorocarbene
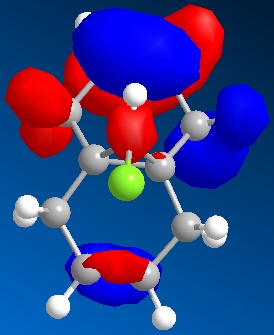
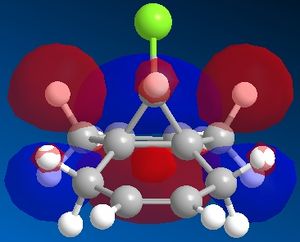
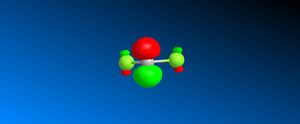
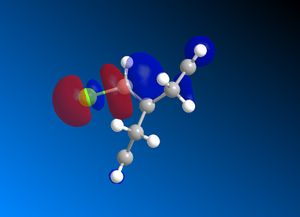
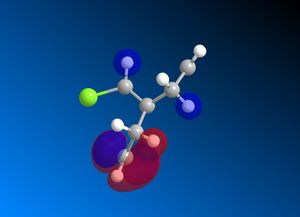
Reactant molecule 1 was optimized using the MM2 force field (Table 3). The HOMO-1, HOMO, LUMO, LUMO+1 and LUMO+2 molecular orbitals were then calculated using the MOPAC interface (shown right).
The reaction of reactant molecule 1 with dichlorocarbene is similar to electrophilic attack by the dichlorocarbene molecule on the HOMO so by studying the image created via the MOPAC interface of the HOMO we can see that there is a greater amount of electron density located around the C=C double bond that is syn to the C-Cl bond and hence this is where the reactant molecule will be attacked by the electrophilic dichlorocarbene.
| Energy Type | Reactant Molecule |
|---|---|
| Stretch | 2.5807 |
| Bend | 19.78 |
| Stretch-Bend | 0.16747 |
| Torsion | 32.086 |
| Non-1,4-VdW | -4.4284 |
| 1,4-VdW | 24.27 |
| Dipole-Dipole | 0.47018 |
| Total | 74.926 |
Reactant molecule 2 (right) is the same as Reactant molecule 2 but with the anti double bond hydrogenated to give a C-C single bond to produce a mono-alkene. The stretch frequencies of the C-Cl, syn-C=C and anti-C=C bonds of reactant molecule 1 and 2 were then compared (Table 4) using the IR spectra obtained with Gaussian using the .chkf file obtained after optimizing the molecules using Semi-empirical Molecular Orbital Theory.
A peak at a lower frequency for the same bond indicates a longer and hence weaker bond. If we compare the syn and anti C=C bonds for Reactant molecule 1 we can see that the the anti C=C bond has a lower frequency and hence is a weaker bond which suggests that this bond is more reactive than the syn-C=C bond. This is the opposite to what is suggested previously by analysing the HOMO. This means that the anti-C=C bond is being destabilised in some way probably by donation of electron density into its antibonding orbital from other atoms in the molecule.
The frequency of the C-Cl bond in the 2 molecules is different. The frequency is higher in the monoalkene which suggests a weaker bond therefore the presence of both the C=C double bonds stabilises the C-Cl bond.
The frequency of the syn-C=C bond in the 2 molecules is also different. In this case the bond is weaker in the dialkene.
| Bond | Reactant Molecule 1 Freq. | Reactant Molecule 2 Freq. |
|---|---|---|
| C-Cl | 772.545 | 782.303 |
| syn C=C | 1760.99 | 1757.33 |
| anti C=C | 1740.87 |
Structure based Mini project using DFT-based Molecular orbital methods
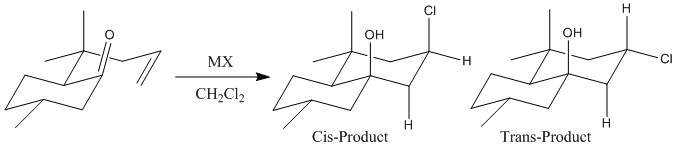
Syn- and Anti-Selective Prins Cyclizations of delta,epsilon-Unsaturated Ketones
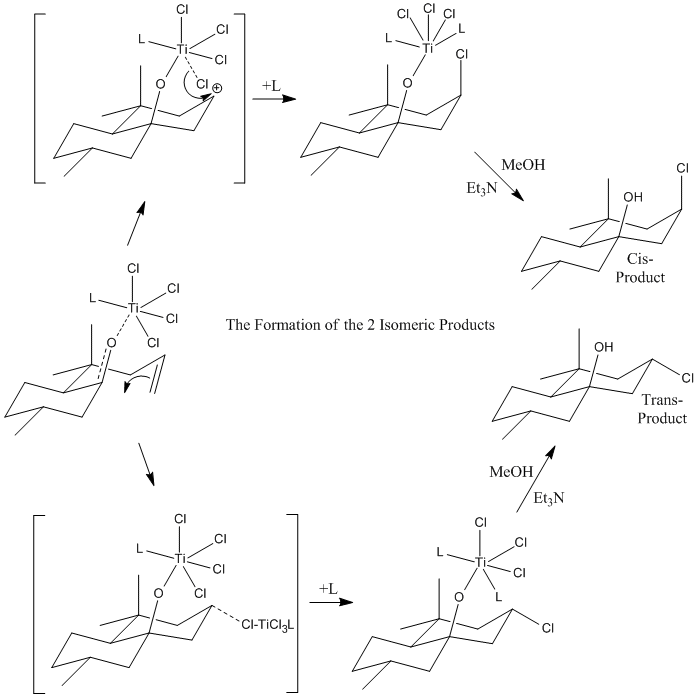
The Prins Reaction was investigated by predicting the 13C NMR Spectrum of a compound; the 3J H-H couplings and the IR Spectrum of the 2 isomers formed in a particular study of the reaction by R. B. Miles and C. E. Davis1. The Prins reaction consists of the electrophilic addition of a ketone/aldehyde to an alkene/alkyne. The outcome of a Prins reaction depends greatly on the reaction conditions as the intermediates formed are influenced by varying environments e.g. the oxonium ion formed can react with nucleophiles if present in the reaction mixture.
The stereochemistry of the product depends upon how the electrophilic addition of the ketone/aldehyde across the carbon-carbon double bond takes place i.e. if syn addition takes place then a cis product is formed and if an anti addition takes place a trans product is formed. It is often found that the trans product is favourable due to Prins reactions being anti-stereoselective but this outcome can be influenced by the addition of certain lewis acids into the reaction mixture which cause neighbouring group participation and hence the formation of a cyclic, lower energy transition state and hence, syn-addition is favoured and the cis-product dominates.
The starting product and the 2 possible isomers were optimized using MM2 and MMFF94 methods (Table 5). Strangely, the two different methods of optimizing the conformations gave opposite results in terms of which isomer is the most stable. Using MM2 the cis-isomer has the lowest energy whereas with MMFF94, the trans-isomer is calculated to have the lowest energy. This highlights the fact that purely molecular modelling methods of determining the lowest energy conformations of isomers is not reliable enough to use in calculations where very accurate results are desired.
| Molecule | MM2 | MMFF94 |
|---|---|---|
| Initial | 78.896 | 154.16 |
| cis-isomer | 80.757 | 183.11 |
| trans isomer | 87.361 | 165.02 |
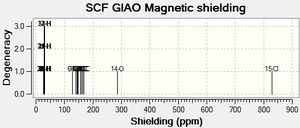
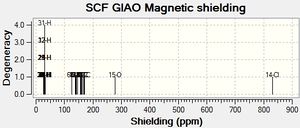
Predicting the 13C NMR Spectrum of the 2 isomers
The molecules were optimized using MM2 Molecular Mechanics and then using MOPAC via the SCAN system. The output files were then edited so that once resubmitted the NMR of the 2 isomers would be predicted. The results are shown below in Table 7 and the 2 graphs are also shown below.
| Carbon Number | Caluclated Shift (ppm) |
|---|---|
| C6 (Bonded to O) | 127.048 |
| C12 (Bonded to Cl) | 140.058 |
| C5 (Adjacent to C bonded to O) | 143.895 |
| C4 (Adjacent to C bonded to O) | 144.979 |
| C11 (Adjacent to C bonded to Cl) | 146.731 |
| C13 (Adjacent to C bonded to Cl) | 148.232 |
| C2 | 156.739 |
| C9 | 159.825 |
| C8 | 160.534 |
| C3 | 163.995 |
| C10 | 167.289 |
| C7 | 168.415 |
| C1 | 169.083 |
| Carbon Number | Caluclated Shift (ppm) |
|---|---|
| C6 (Bonded to O) | 126.329 |
| C12 (Bonded to Cl) | 138.8 |
| C13 (Adjacent to C bonded to Cl) | 140.103 |
| C5 (Adjacent to C bonded to O) | 141.531 |
| C11(Adjacent to C bonded to Cl) | 142.834 |
| C4(Adjacent to C bonded to O) | 146.06 |
| C2 | 156.422 |
| C8 | 157.539 |
| C9 | 160.641 |
| C3 | 13.619 |
| C7 | 168.739 |
| C1 | 168.956 |
| C10 | 169.886 |
The literature values ranged between 22.0 to 72.0ppm for the cis-isomer and 21.4 to 73.8ppm for the trans-isomer. Clearly there has been some sort of error whilst calculating the NMR shifts as my results range between 127.048 to 169.083ppm for the cis isomer and 126.329 to 169.886ppm for the trans isomer which is approximately 100ppm away for both isomers(very far out). Although this is the case, the results for the 2 isomers can be compared to each other and the shifts of the carbon atoms in the same molecule are, to some extent, also comparable i.e. the carbon atoms bonded to the electronegative oxygen and chlorine atoms are the most deshielded in the spectrum.
The shielding values for the carbon atoms adjacent to the carbon atom bonded to the Cl are both approximately 6ppm greater for the cis-isomer than the same atoms in the trans isomer and hence this could be used to distinguish between the 2 compounds. The shielding value for the C atom bonded directly to Cl is approximately 2ppm greater in the cis isomer aswell which could also be used to distinguish between the compounds. Of course the difference in shifts would be alot different if the results were closer to the literature values.
Predicting the 3J H-H couplings of the 2 isomers
Using the MOPAC optimized data, the files for the 2 isomers were converted into .jmol files and Janocchio was used to find the 3J H-H coupling of the hydrogen atom bonded to the same carbon atom as the chlorine with the hydrogens bonded to the adjacent carbons (Table 8).
| Hydrogen | Predicted cis Value | Predicted trans Value |
|---|---|---|
| 1 | 2.9479 | 3.9380 |
| 2 (axial) | 3.9039 | 11.5347 |
| 3 (adjacent to C-O) | 2.6969 | 4.1612 |
| 4 (adjacent to C-O, axial) | 4.0290 | 11.5231 |
As we can see the axial hydrogens have much larger coupling constants with the H bonded to the same carbon as the chlorine for the trans isomer than any of the other hydrogens. Therefore these values could be used to distinguish between the 2 isomers. However, this may not be the case as the NMR data returned from SCAN is not very accurate as seen in the previous section on NMR
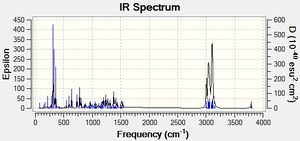
Predicting the IR Spectra of the 2 isomers
After optimizing the isomers using MM2 and MOPAC the .chkf files were edited to give the IR spectrum and submitted to SCAN.
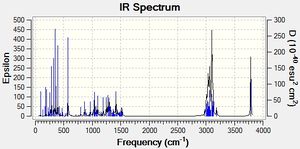
| Vibration | Predicted Value | Literature Value |
|---|---|---|
| O-H Stretch | 3786.26 | 3592 |
| C-H Stretch (H on same atom as Cl) | 3166.88 | 3479, 2947, 2867 |
| C-H Stretches | 2975.36 - 3182.53 | 3479, 2947, 2867 |
| C-H Bends | 327.794-1528.35 | 1370, 1455 |
| O-H Bend | 318.324 | 650-770 |
| Vibration | Predicted Value | Literature Value |
|---|---|---|
| O-H Stretch | 3792.83 | 3592 |
| C-H Stretch (H on same atom as Cl) | 3139.84 | 3479, 2947, 2867 |
| C-H Stretches | 2995.18 - 3160.45 | 3479, 2947, 2867 |
| C-H Bends | 79.4131-1535.91 | 1370, 1455 |
| O-H Bend | 311.338- 354.214 | 650-770 |
The predicted vibrations of the isomers were compared to the literature values. For both isomers, the predicted vibration ranges for the C-H bends and stretches contained the literature values. However, the the values for the O-H stretches and bends, are not. The values predicted for the O-H bends are approximately 300cm-1 out for both isomers and the predicted value for the O-H stretches are approximately 200-1 out for both isomers.
As these isomers are different due to the orientation of the Cl atom it may be useful to compare the C-H stretches for the hydrogen atom bonded to the same carbon as the chlorine atom to distinguish between the 2 isomers. In the predicted values, the value for this stretch is approximately 27cm-1 higher for the cis isomer compared to the trans-isomer.
Unfortunately both the literature and the predicted values did not contain values for the C-Cl stretch which may be the best tool for distinguishing between the isomers using IR spectroscopy.
References
1. R. B. Miles, C. E. Davis, J. Org. Chem., 2006, Vol. 71, No. 4, 1493-1501. DOI:10.1021/jo052142n
Notes
An attempt was made to predict the optical rotation of the 2 isomers but once i got the files, i had no idea how to find the values!