Gp316Inorganic
BH3
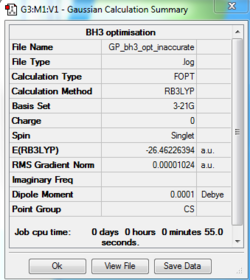
3-21G Optimisation Summary
Summary Table
Item Value Threshold Converged? Maximum Force 0.000217 0.000450 YES RMS Force 0.000105 0.000300 YES Maximum Displacement 0.000759 0.001800 YES RMS Displacement 0.000442 0.001200 YES
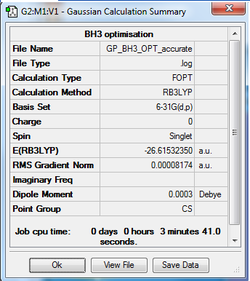
6-31G Optimisation Summary
Summary Table
Item Value Threshold Converged? Maximum Force 0.000203 0.000450 YES RMS Force 0.000098 0.000300 YES Maximum Displacement 0.000867 0.001800 YES RMS Displacement 0.000415 0.001200 YES
BH3 belongs to the D3h point group but due to the limitations of the Gaussian software it has been assigned as CS. D3h symmetry was imposed on the optimised molecule before carrying out a frequency analysis.
Frequency Analysis
Link to BH3 frequency analysis log file:here
Low frequencies --- -0.4059 -0.1955 -0.0054 25.3480 27.3326 27.3356 Low frequencies --- 1163.1913 1213.3139 1213.3166
BH3 molecule |
BH3 Vibrations
| Wavenumber (cm-1) | Intensity (arbitrary units) | Symmetry | IR active? | Type |
|---|---|---|---|---|
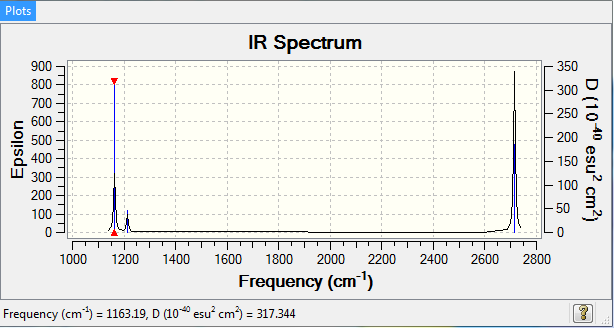
| 1163 | 93 | A2'' | yes | out-of-plane bend |
| 1213 | 14 | E | very slight | bend |
| 1213 | 14 | E | very slight | bend |
| 2582 | 0 | A1' | no | symmetric stretch |
| 2715 | 126 | E' | yes | asymmetric stretch |
| 2715 | 126 | E' | yes | asymmetric stretch |
BH3 IR Spectrum
Fewer than 6 peaks in the IR spectrum even though there are 6 vibrations as some of the vibrations will not be accompanied by a change in dipole moment (a requirement in order to be IR active).
Ng611 (talk) 19:06, 6 June 2018 (BST) That accounts for 1x missing IR peak, what about the other two that are missing in the spectrum?
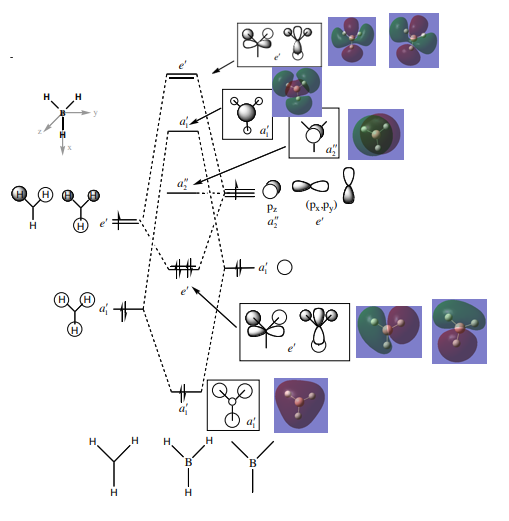
BH3 MO Diagram
In general, the MOs almost mirror the LCAOs. The a1' MO shows that the MO is diffuse over the entire molecule as opposed to the more atom localised image presented by LCAO. The e' LCAO derived from the px AO suggests that this MO should be equal in bonding and antibonding character. However, the MO shows that this is not the case with one side being more diffuse than the other. Overall, the similarity between the LCAO diagrams and the real MOs suggest that qualitative MO theory is more than accurate enough for our purposes here.
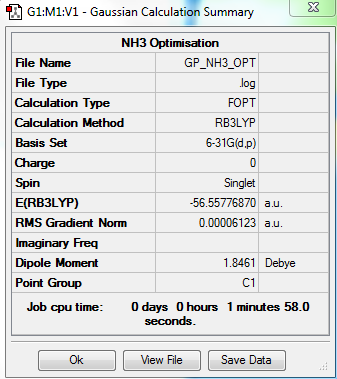
NH3
6-31G Optimisation Summary
Summary Table
Item Value Threshold Converged? Maximum Force 0.000100 0.000450 YES RMS Force 0.000050 0.000300 YES Maximum Displacement 0.000177 0.001800 YES RMS Displacement 0.000113 0.001200 YES
NH3 belongs to the C3V point group but due to the limitations of the Gaussian software it has been assigned as C1. C3V symmetry was imposed on the optimised molecule before carrying out a frequency analysis.
Link to NH3 frequency analysis log file:here
Low frequencies --- -33.1843 -32.6528 -32.6527 -0.0012 -0.0012 0.0024 Low frequencies --- 1088.9832 1693.8057 1693.8057
NH3 molecule |
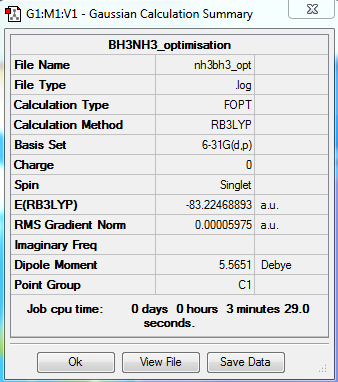
NH3BH3
6-31G Optimisation Summary
Summary Table
Item Value Threshold Converged? Maximum Force 0.000122 0.000450 YES RMS Force 0.000058 0.000300 YES Maximum Displacement 0.000513 0.001800 YES RMS Displacement 0.000296 0.001200 YES
Link to NH3BH3 frequency analysis log file:here
Low frequencies --- -0.0012 -0.0011 0.0011 17.1488 17.8526 37.4337 Low frequencies --- 265.8896 632.2187 639.3610
NH3BH3 molecule |
E(BH3)= -26.61532350
E(NH3)= -56.55776870
E(NH3BH3)= -83.22468893
Dissociation Energy = 0.05159673 au
Dissociation Energy = 135 kJ/mol
Thus it is a relatively weak bond, lower in energy than that of comparable single bonds shown in the table below. [1]
| Bond | Energy (kJ/mol) |
|---|---|
| B-N | 135 |
| O-O | 146 |
| N-N | 160 |
| C-C | 347 |
Ng611 (talk) 19:08, 6 June 2018 (BST) Correct calcualtion and good consideration given to the accuracy of the reported result. You should endevour to use peer-reviewed sources where possible.
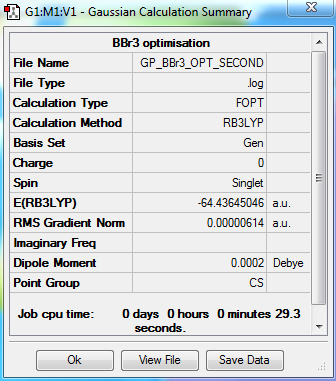
BBr3
Optimisation Summary
Summary Table
Item Value Threshold Converged? Maximum Force 0.000010 0.000450 YES RMS Force 0.000007 0.000300 YES Maximum Displacement 0.000045 0.001800 YES RMS Displacement 0.000032 0.001200 YES
BBr3 belongs to the D3h point group but due to the limitations of the Gaussian software it has been assigned as CS. D3h symmetry was imposed on the optimised molecule before carrying out a frequency analysis.
Link to BBr3 frequency analysis log file:here
Low frequencies --- -1.9018 -0.0001 0.0001 0.0002 1.5796 3.2831 Low frequencies --- 155.9053 155.9625 267.7047
BBr3 molecule |
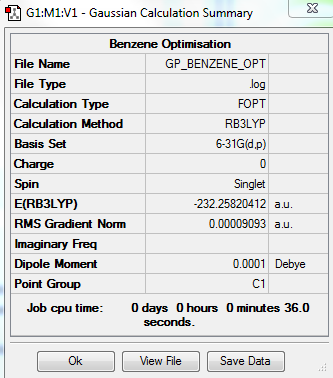
Benzene
Optimisation Summary
Summary Table
Item Value Threshold Converged? Maximum Force 0.000198 0.000450 YES RMS Force 0.000082 0.000300 YES Maximum Displacement 0.000849 0.001800 YES RMS Displacement 0.000305 0.001200 YES
Benzene belongs to the D6h point group but due to the limitations of the Gaussian software it has been assigned as C1. D6h symmetry was imposed on the optimised molecule before carrying out a frequency analysis.
Link to benzene frequency analysis log file:here
Low frequencies --- -13.7810 -12.9763 -11.9029 -0.0002 0.0001 0.0009 Low frequencies --- 414.0699 414.1914 620.9703
Benzene molecule |
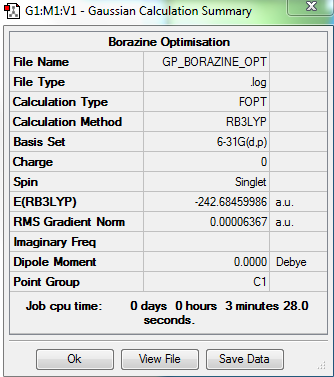
Borazine
Optimisation Summary
Summary Table
Item Value Threshold Converged? Maximum Force 0.000084 0.000450 YES RMS Force 0.000033 0.000300 YES Maximum Displacement 0.000276 0.001800 YES RMS Displacement 0.000077 0.001200 YES
Borazine belongs to the D3h point group but due to the limitations of the Gaussian software it has been assigned as C1. D3h symmetry was imposed on the optimised molecule before carrying out a frequency analysis.
Link to borazine frequency analysis log file:here
Low frequencies --- -7.5576 -0.0008 -0.0002 0.0008 7.2648 13.2011 Low frequencies --- 288.5679 290.5561 404.2330
Borazine molecule |
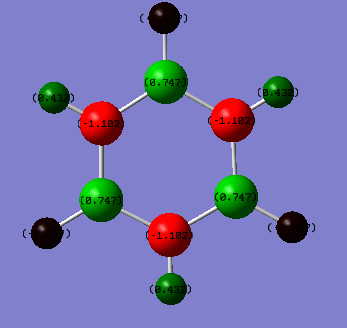
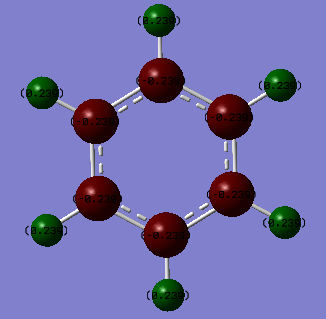
Borazine:Benzene Charge Analysis
| Borazine | Benzene |
|---|---|
 |
|
Formal Charges
| Benzene | Formal Charge (NPH) |
|---|---|
| C | -0.239 |
| H | 0.239 |
| Borazine | Formal Charge (NPH) |
|---|---|
| B | 0.747 |
| N | -1.102 |
| H (B-H) | -0.077 |
| H (N-H) | 0.432 |
Benzene is D6h symmetric and shows just two charge environments. All 6 carbon atoms are equivalent and hence carry the same charge and the same is seen for the 6 hydrogen atoms which are in identical environments so carry the same charge. The electronegativity of carbon is 2.55 whilst that of hydrogen is 2.20.[2] As carbon is the more electronegativte atom, it draws electron density within the C-H bond towards itself resulting in the negative charge whilst the hydrogen carries a positive charge.
With borazine, charge is distributed unevenly between the boron and nitrogen atoms. Nitrogen is more electronegative than Boron (3.04 compared to 2.04 respectively) and hence carries the negative charge.[2] Another consequence of the difference in electronegativities is the B-H hydrogens being slightly negative in charge whilst the N-H hydrogens are positive. As nitrogen is significantly more electronegative than hydrogen it draws the electron density away from the hydrogen atom. Whereas hydrogen and boron have very similar electronegativities resulting in only very small charge values across the B-H bonds. Boron is slightly less electronegative than hydrogen and thus the B-H hydrogens are the only ones to carry a negative charge in these two examples.
Ng611 (talk) 19:11, 6 June 2018 (BST) Good discussion of the effects of electronegativity on the overall charge distribution. What do the partial charges sum to, and how does borazine's D3H point group affect the distribution of charge.
Comparing MOs
| Borazine | Benzene | Discussion |
|---|---|---|
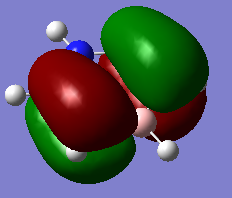 MO21 MO21 |
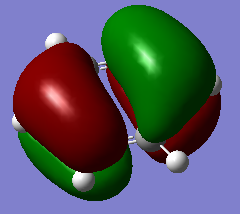 MO21 MO21 |
Both MOS are pi-bonding with mostly p-atomic orbital contributions. While the benzene MO is symmetric (E1g), the borazine MO is not. Again this is due to distortions in electron density as a result of the differing energies of the boron and nitrogen atoms. As nitrogen is lower in energy and this is a bonding MO, we expect a greater contribution to the MO from the nitrogen orbitals. Ng611 (talk) 19:14, 6 June 2018 (BST) Good discussion. It's also worth pointing out that that C3 symmetry in borazine has a very strong effect on the symmetry of these orbitals. |
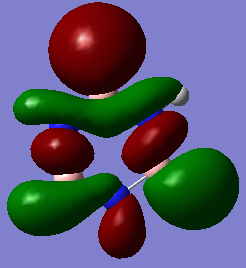 MO19 MO19 |
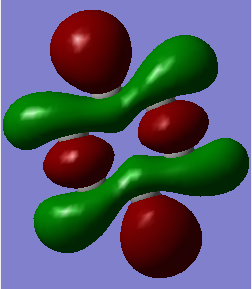 MO19 MO19 |
Here, the benzene sigma antibonding MO is symmetric due to the equivalency of the carbon atoms. This is not the case for the borazine antibonding sigma MO; boron is higher in energy than nitrogen and so has a greater contribution to the antibonding MO. Hence this MO is distorted towards the boron atoms. When considering atomic orbital contributions, we can see the hydrogen s-orbital contributions at the top and bottom of each MO and carbon p-orbitals dominating in the central region as shown in the benzene schematic. 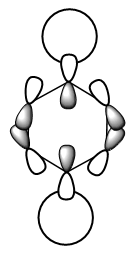 Ng611 (talk) 19:14, 6 June 2018 (BST) I like this schematic a lot. I'd suggest making one for all three of your MO diagrams. |
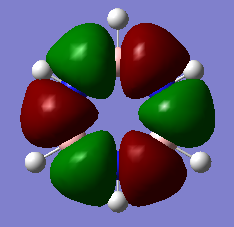 MO15 MO15 |
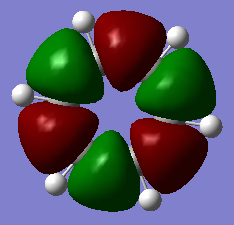 MO14 MO14 |
The high energy, sigma bonding and p-orbital dominated MOs for borazine and benzene are very similar. There is a subtle difference between the two, while the orbitals in the benzene MO are completley symmetric, those of borazine differ slightly due to the different electronic contributions from the boron and nitrogen atoms to the MO. The high symmetry of the benzene MO is reflective of its D6h point group. |
Ng611 (talk) 19:15, 6 June 2018 (BST) Some good points made in this MO analysis, although they're somewhat inconsistently applied. As a minimum: for every orbital you compare you should consider the overall character of the orbital (sigma, pi etc) and the symmetry label of the orbital, relative contributions from AOs (and why the relative values are the way they are), how the symmetry/point group of the molecules affects the shapes of the orbitals, and the constituent AOs (i.e. 2px/y/z, 1s, etc.). You've done different bits for each orbital comparison, but not all of them for every orbital.
Aromaticity
The concept of aromaticity is used to describe the unusual stability of molecules with alternating double bonds such as benzene. Specifically, to be aromatic the molecule must: be planar, cyclic, have a contiguous ring of p-orbitals and contain 4n+2 pi-electrons. This final rule derives from Huckel theory. Aromaticity can be illustrated using MO theory by taking a simple example, benzene. Rather than show localised areas of electron density, the MO is delocalised across the whole molecule. Simply, this could be thought of as the overlap of neighbouring singly-occupied pz orbitals resulting in delocalisation.
However, this simple picture breaks down when considering larger molecules with fused rings such as pyrene.[3] Whilst containing 4n pi electrons, it is still an aromatic molecule, breaking the Huckel rule of aromaticity. Aromatic compounds need not be planar; for example pyrenophane is not planar yet still aromatic.[4]. Perhaps an even more extreme example is that of closo-boranes which are not at all planar yet still aromatic. Thus a more sophisticated model is required; currently an active area of research, a consensus has not yet been reached. It has been postulated that the sigma structure is a greater contributor to aromaticity than previously suspected. [5]
Ng611 (talk) 19:17, 6 June 2018 (BST) You hint in the penultimate sentence about a more sophisticated model. This is actually a really important part of this discussion. How does this modern view of aromaticity differ from the previous one? What are some aspects of this model? More detail in this final piece would have strengthed this section greatly.
Ng611 (talk) 19:19, 6 June 2018 (BST) A good overall report. Some additional discussion in your second section would have made it near-perfect. Well done!
References
- ↑ http://butane.chem.uiuc.edu/cyerkes/Chem104ACSpring2009/Genchemref/bondenergies.html
- ↑ 2.0 2.1 https://www.angelo.edu/faculty/kboudrea/periodic/trends_electronegativity.htm
- ↑ Roberts, John D.; Streitwieser, Andrew, Jr.; Regan, Clare M. (1952). "Small-Ring Compounds. X. Molecular Orbital Calculations of Properties of Some Small-Ring Hydrocarbons and Free Radicals". J. Am. Chem. Soc. 74 (18): 4579–82. doi:10.1021/ja01138a038
- ↑ Boazhong Zhang, Gregory P.Manning, (2008).Nonplanar Aromatic Compounds. 9. Synthesis, Structure, and Aromaticity of 1:2,13:14-Dibenzo[2]paracyclo[2](2,7)- pyrenophane-1,13-diene".Org. Lett., 10 (2), pp 273–276 DOI: 10.1021/ol702703b
- ↑ Palusiak, M. and Krygowski, T. (2007), Application of AIM Parameters at Ring Critical Points for Estimation of p-Electron Delocalization in Six-Membered Aromatic and Quasi-Aromatic Rings. Chemistry - A European Journal, 13:7996-8006. doi:10.1002/chem.200700250