Gmj
Molecular Reaction Dynamics: Applications to Triatomic systems
Introduction
A triatomic system, which is made up of a single atom A and a diatomic molecule B-C, is investigated in order to understand the molecular reaction dynamics. The reaction between an atom A and a molecule B-C will only happen under certain condition. The reaction trajectory is tested by MATLAB program. Setting different initial distances and momenta of A-B and B-C would give different reaction trajectories which indicate the reactivity of collision. Based on the reaction trajectory, transition state and activation barrier could be found. The energy applied (momentum) must be greater than the activation barrier to promote reaction. However, this is not enough for reaction. Based on energetics of reaction, vibrational or translational energy is more efficient to promote the reaction.
Reaction 1: H+H2 system
In this reaction, H atom(HA) will collide with H2 molecules(HB-HC) and form a new H2 molecules(HA-HB) and a new H atom(HC).
The distance between HA and HB is define as r1, and the momentum of them is defined as p1.The distance between HB and HC is define as r2, and the momentum of them is defined as p2. All of them will change with time, and the initial condition is:
r1 = 2.30 Å, p1 = -2.70 Ns
r2 = 0.74 Å, p2 = 0.00 Ns
Dynamics of the transition state and minimum of potential energy
The transition state of a reaction is a configuration with the highest potential energy along the reaction coordinates and transition state determine whether the reaction is reversible or not and the rate of reaction. If the energy of the transition state is extremely high, the reaction will be irreversible and rate of reaction will be relatively slow; vice versa. Hence, finding a transition state is very important for a reaction.
In order to find transition state and the minimum of potential energy, the gradient of the potential should be 0.
However those two states could be distinguished by second derivative .
For transition state, it is a saddle point in the reaction path[1], which means that it is a minimum point in one axis and is a maximum point in another axis. For any reaction, it will follow the lowest potential pathway.Hence it is a minimum point in potential axis. But transition sate is the maximum potential energy along the reaction coordinates; so transition state is a saddle point and the second derivative at transition state should be negative.
For the minimum point of potential energy, it is a local minimum. Hence, the second derivative at minimum point should be positive.
Nf710 (talk) 16:03, 26 May 2017 (BST) You find the reactio coordinate by changing the basis into the normal modes of the system.
Determination of transition state
The energies of the reactants and products are same in this reaction and the potential surface is symmetric.Hence, the transition state should have r1 = r2 = rts
In order to find out rts, momenta are set to be zero.( p1 = p2=0 Ns). This made the system stay in transition state.
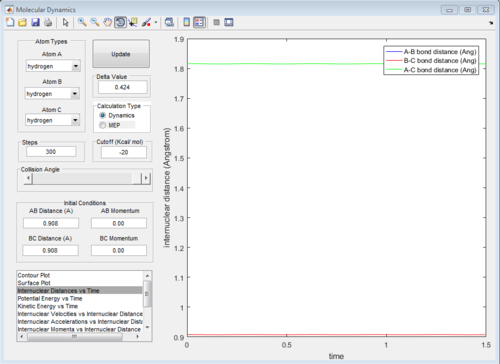
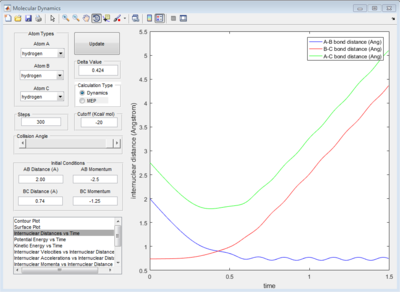
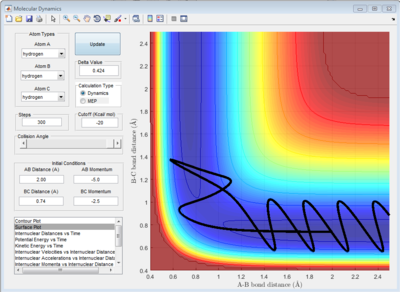
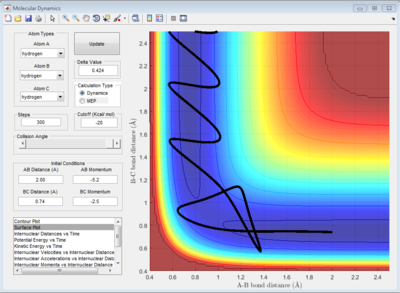
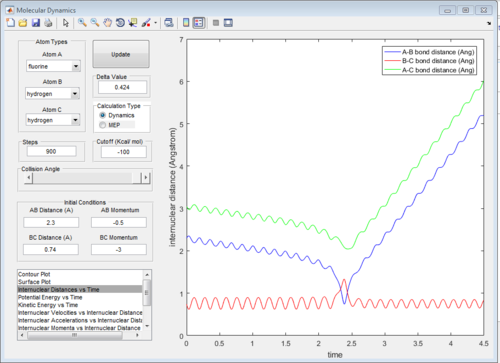
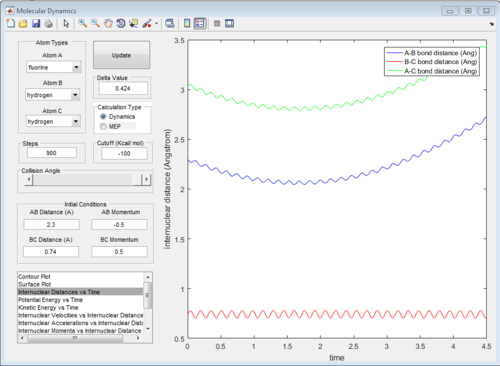
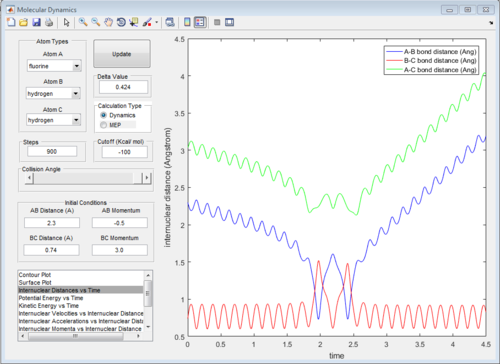
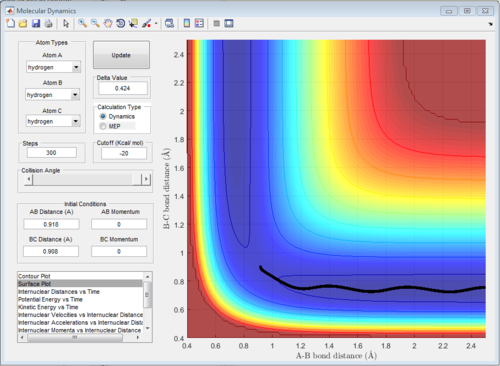
According to Fig.1 ,at r1 = r2 = 0.908 Å, the internuclear distance does NOT oscillate very much with time, which indicates that rts is around 0.908 Å.
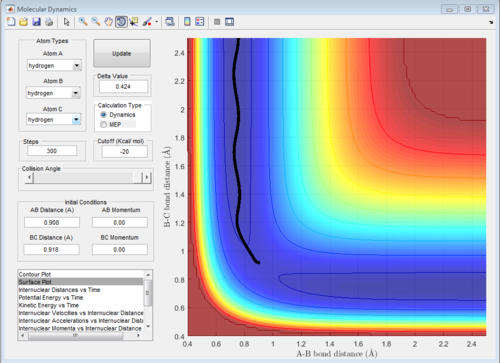
Minimum energy path(MEP) and dynamic path
Minimum energy path(MEP) is a special trajectory that the velocity is reset to zero in every time step. Moreover, MEP is used for a given reaction and it did not take realistic motion of atoms into account (e.g. vibrational energy). In other words, MEP only follows the lowest energy reaction path in the surface plot.
The initial condition:
r1 = rts +0.01 = 0.918 Å, p1 = 0.00 Ns
r2 = rts = 0.908 Å, p2 = 0.00 Ns
 |
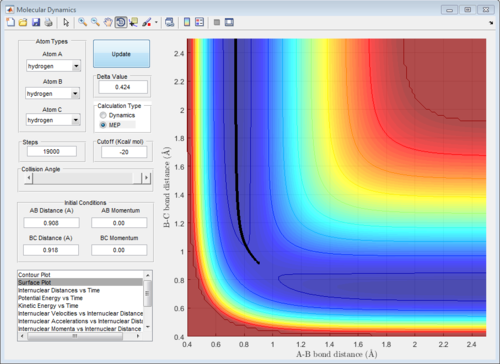 |
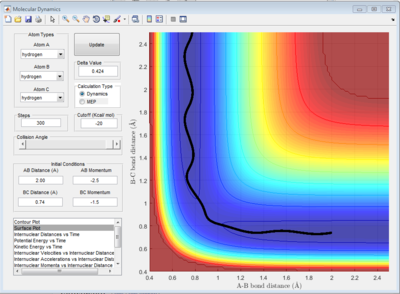
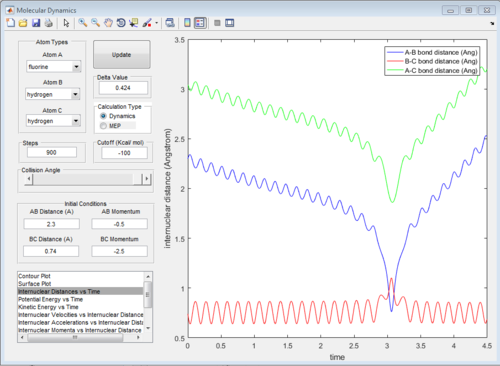
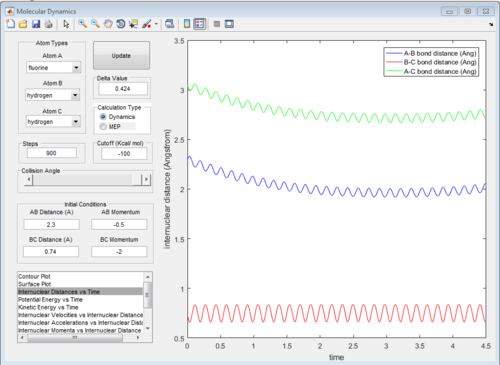
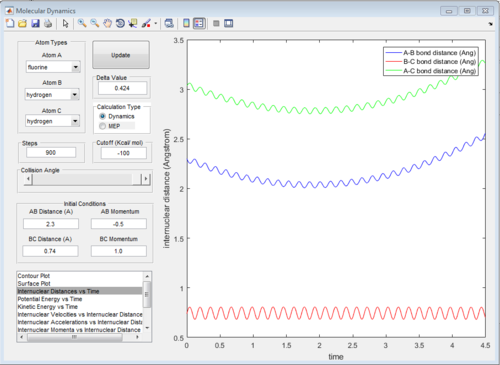
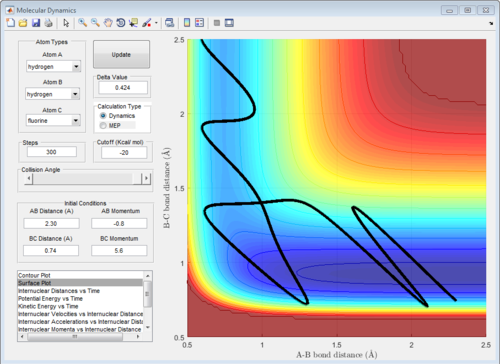
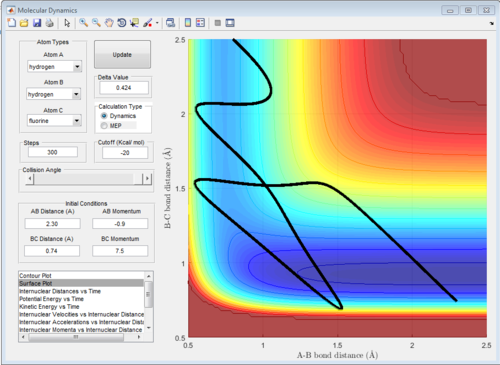
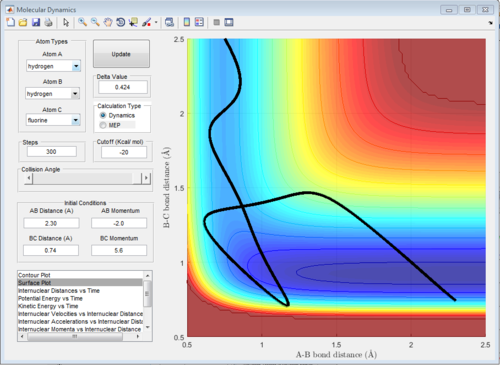
The potential surface diagrams under dynamic method and under MEP method are showed in Fig2. and Fig 3. Comparing those two graphs, MEP method gave a smooth line because MEP method did not consider the realistic motion of molecules (vibration of molecule) and follow the lowest pathway. However, dynamic method considered the vibration of molecule. Hence the line would be fluctuating and do not follow the lowest energy path.
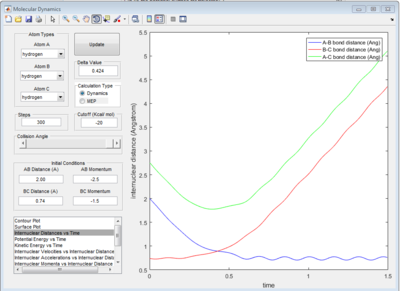
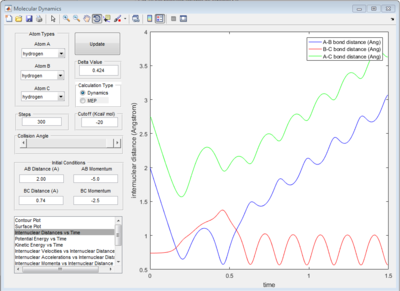
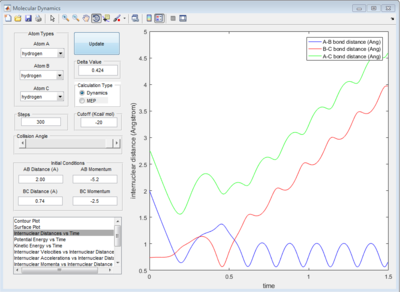
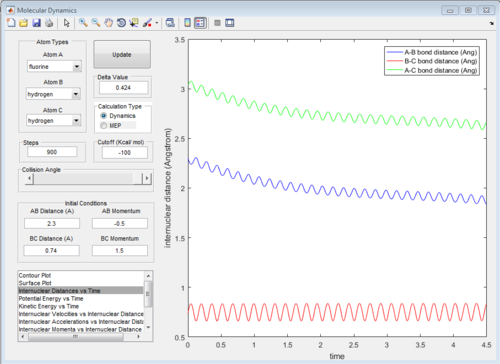
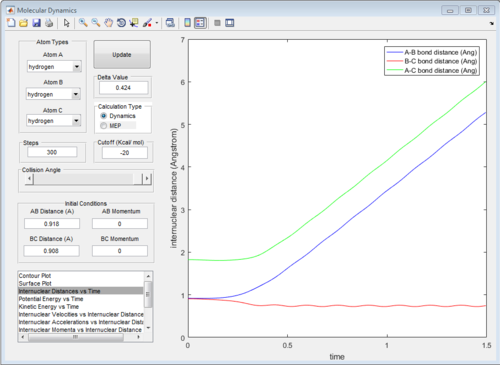
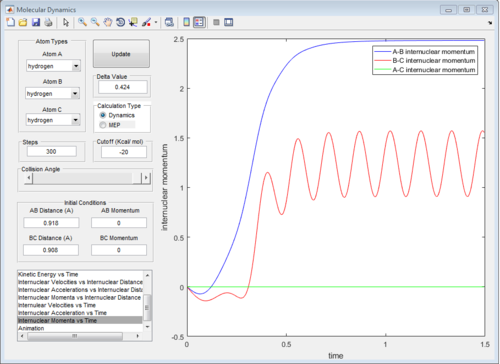
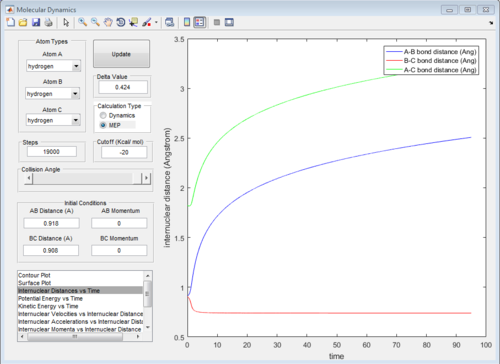
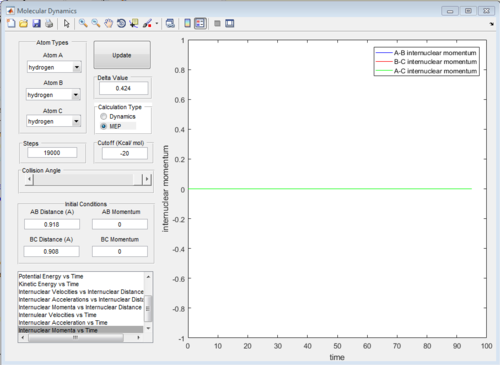
The graph of internuclear distance VS time and the graph of internuclear momentum VS time under dynamic method and under MEP method were showed in Fig. 4-7
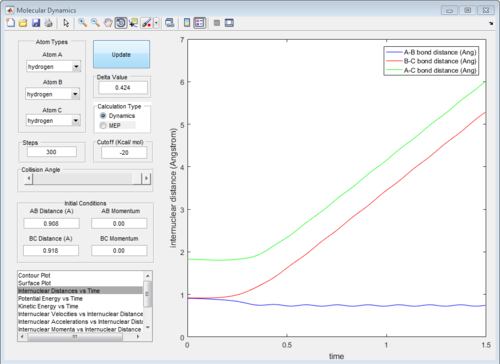 |
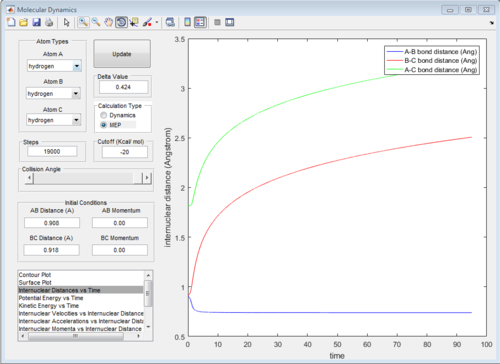 |
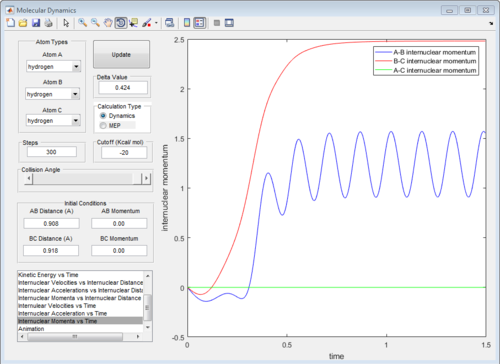 |
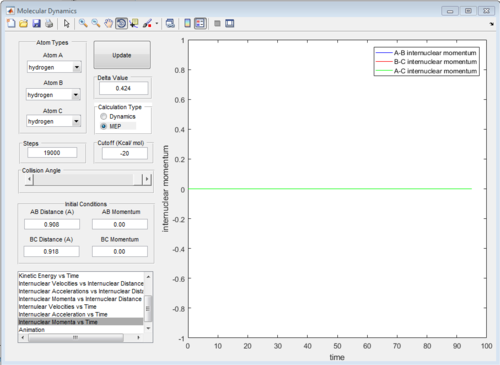 |
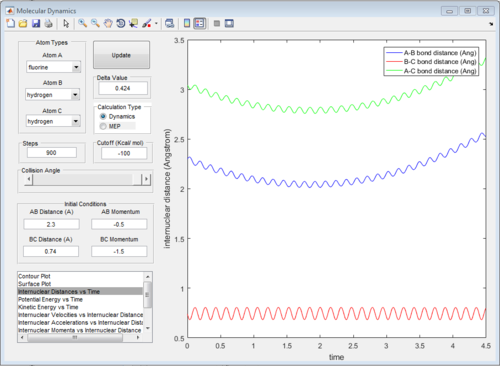
The initial conditions were changed to:
r1 = rts = 0.908 Å, p1 = 0.00 Ns
r2 = rts +0.01 = 0.918 Å, p2 = 0.00 Ns
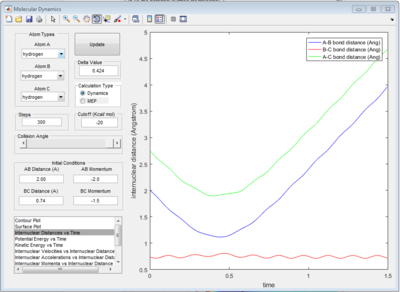
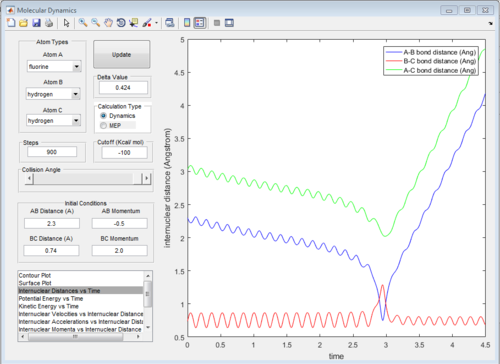
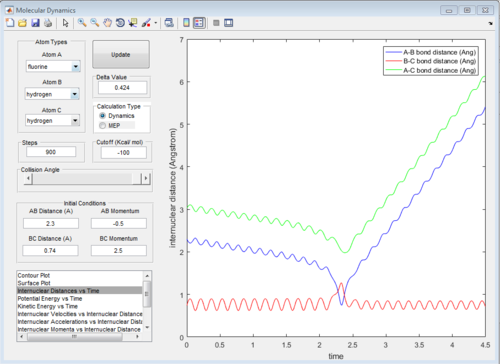
All of the graphs were showed in Fig 8-13.
 |
 |
 |
 |
|
|
 |
 |
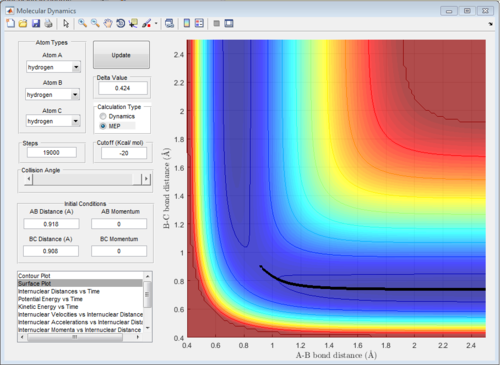
Combining Fig 2.with Fig 8. would give the whole potential surface of this reaction, because Fig8. is the potential surface before reaching the transition state, which means that the B-C bond is going to break and the A-B bond will be formed. However,for Fig. 2, it is the potential surface diagram after the transition state.
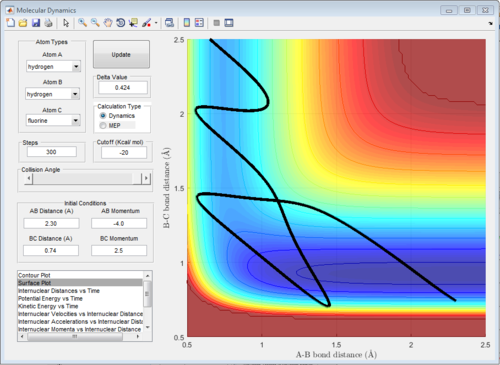
Reactive and unreactive trajectory
Based on the previous calculations, the reaction is only reactive when r1 = 2.0 r2 = 0.74 and p1= -2.5 Ns, -1.5<p2 <-0.8 Ns.
Every reaction has a activation barrier. If the kinetic energy is greater than the activation barrier, the reaction can occur; vice versa. However if the kinetic energy is much larger than the activation energy, it can recross the barrier and this might make reaction unreactive. Therefore, it does not mean that reaction with higher momentum is always reactive.
Transition State Theory
For classical transition state theory (TST), the reaction can only pass through the transition state once from reactant to product or vice versa.It is impossible to recross the transition state. [2]However, in reality, it does not follow the classical transition state theory. For the last two tests in previous section (Fig. 22 and Fig. 23), the reaction recrosses the transition state.
Moreover, the TST rate is proportional to the flux of classical reaction trojectory and it could be calculated by two ways:
1. Maxwell-Boltzmann weighting at a given temperature[3]
2.delta-function weighting to count only trajectories of a given total energy[4]
The rate of reaction should be determined by the transition state in theory. However, this only works at low temperature because at high temperature reaction might not pass through the saddle point (transition state) and the rate of reaction does not depend on the transition states.
The motion of molecules obeys Classical Mechanics. In other words, the vibration is not quantised and tunnelling will not be happened in this reaction. But the influence of quantum mechanics is large, so tunnelling and quantised vibration should be considered.
Nf710 (talk) 16:11, 26 May 2017 (BST) Good understanding of the failures of TST
Reaction 2: F-H-H system
In this three atom system, there are two possible reaction:
1. F atom reacts with H2 molecule to give HF molecule and H atom
2. H atom reacts with HF molecule to give H2 molecule and F atom
Both of those reactions are discussed below. According to those two reactions and Hammond's Postulate[5], their energetics could be distinguished and the activation energy could be calculated.
F + H2 reaction
For this reaction, F atom will collide with H2 molecule and give HF molecule and H atom. Comparing the energies of reactants and products, product will be lower in energy because H-F bond is stronger. The large electronegaticity differences between H and F will give a ionic bond instead of covalent bond and ionic bond is much stronger than covalent bond. The energy of reactant is close to the energy of transition state. Hence this reaction will be exothermic.
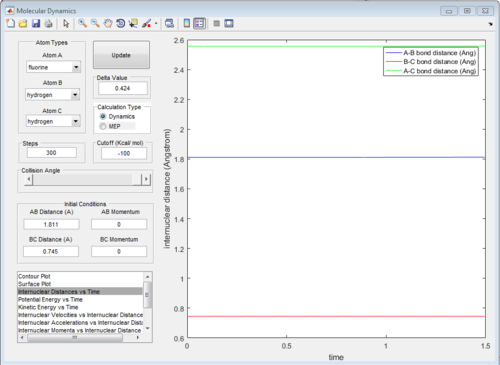
The transition state of this reaction is at:
rFH = 1.811 Å, pFH= 0.0 Ns
rHH = 0.745 Å, pHH= 0.0 Ns
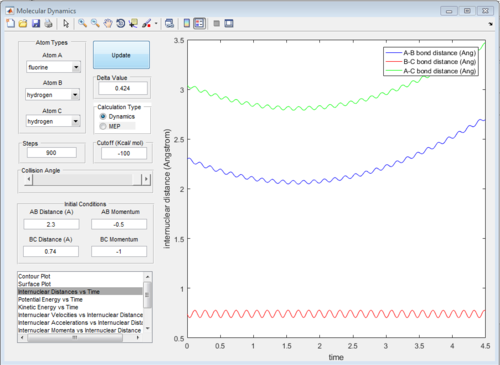
The diagram of internuclear distance VS time is showed in Fig.24 and the surface plot is showed in Fig. 25.
 |
 |
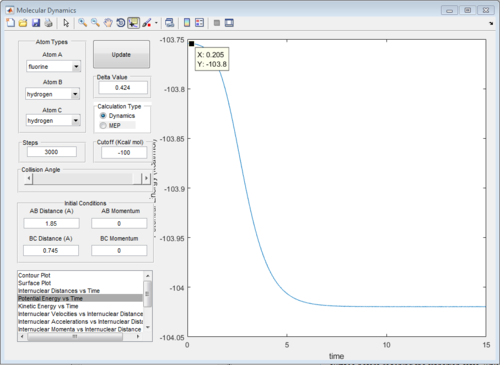
The energy diagrams of transition state and product are showed in Fig. 26 and Fig.27 respectively. The activation energy in this reaction is 0.2kcal/mol
 |
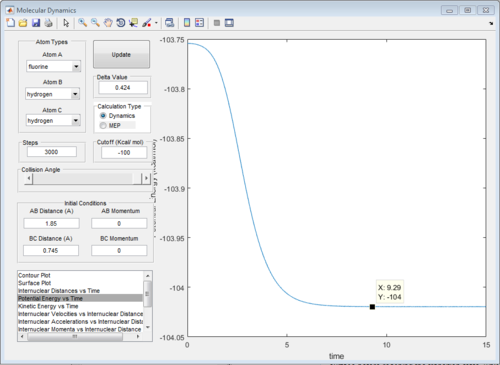 |
H + HF reaction
For this reaction, H atom will collide with HF molecule and give H2 molecule and F atom. Comparing the energies of reactants and products, products will be higher in energy because bond strength of H-F is stronger and HF molecule is more stable than H2 molecule. In other word, the energy of product is closer to the energy of transition state. Hence this reaction will be endothermic.
The transition state of this reaction is at:
rFH = 1.811 Å, pFH= 0.0 Ns
rHH = 0.745 Å, pHH= 0.0 Ns
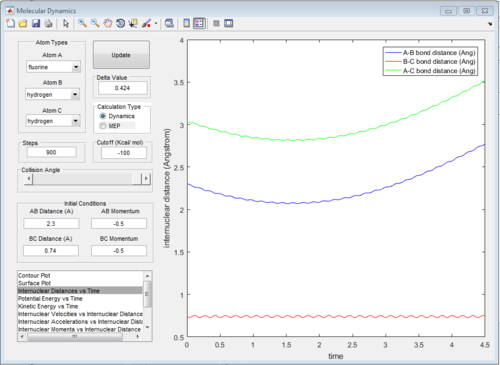
The graph of internuclear distance VS time is showed in Fig.28.
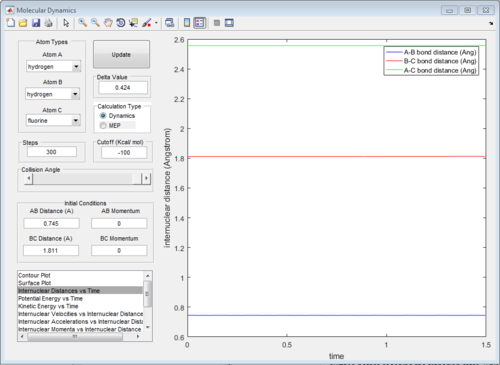
The energy diagrams of transition state and product are showed in Fig. 29 and Fig.30 respectively. The activation energy in this reaction is 30.1 kcal/mol
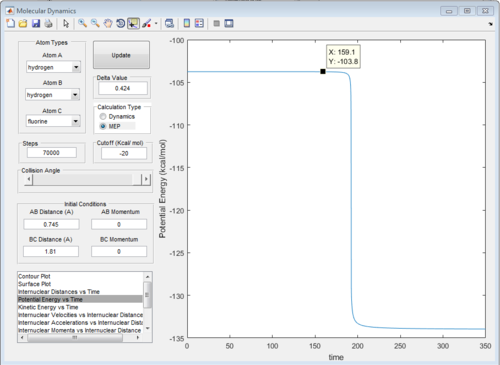 |
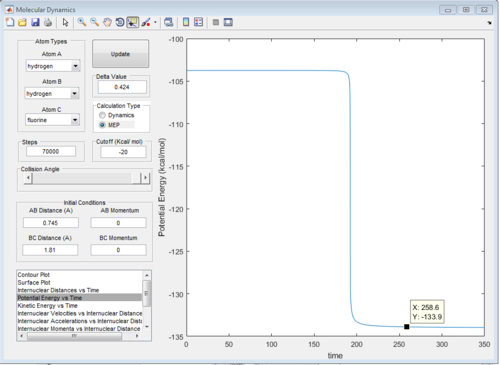 |
Reaction Dynamics
mechanism of F + H2 reaction
The reaction condition set is:
rHF= 2.30 Å, pHF= -2.7 Ns
rHH= 0.75 Å, pHH= 0.0 Ns
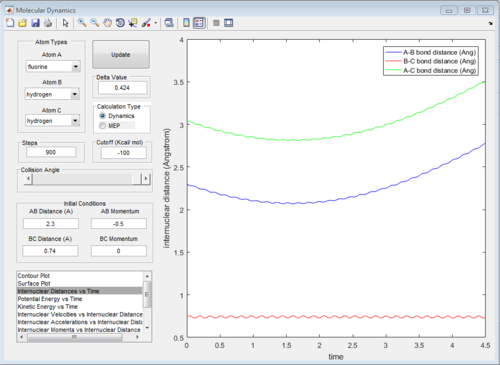
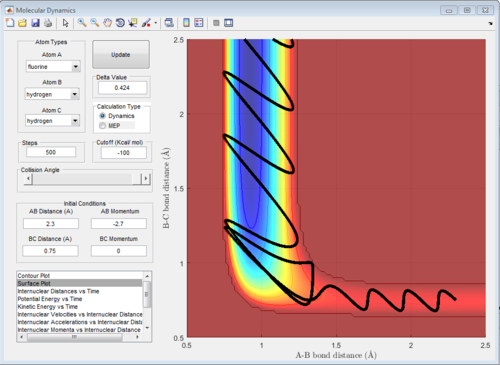
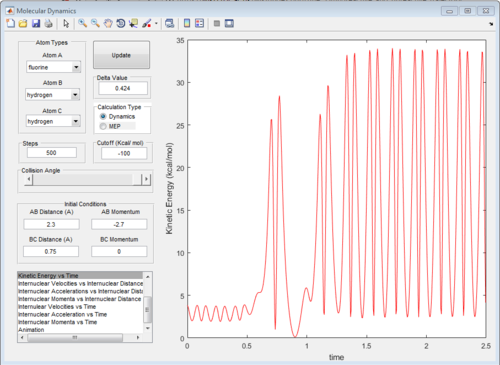
The graphs of internuclear distance VS time, kinetic energy VS time, Potential energy VS time and Potential surface are showed in Fig.31-34.
 |
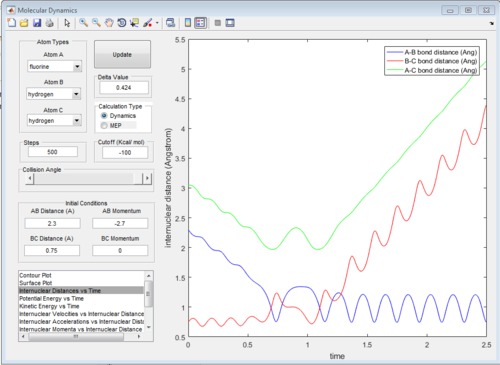 |
Those two graphs suggest that the mechanism of this reaction could be separated into two steps:
1.F atom moves towards the H2 and the bond distance of atom B and atom C decreases. Vibration of H-H bond makes the bond distance between atom B and atom C oscillating. Once, the distance between F atom and H atom reaches certain value (at around transition state), F atom will move back
2.F atom approaches to H2 molecule again and at transition state, H-H bond will be broken and H-F bond will be formed.The vibration of H-F bond is greater than that of H-H bond, so the amplitude of changing in distance between atom A and atom B is greater.
 |
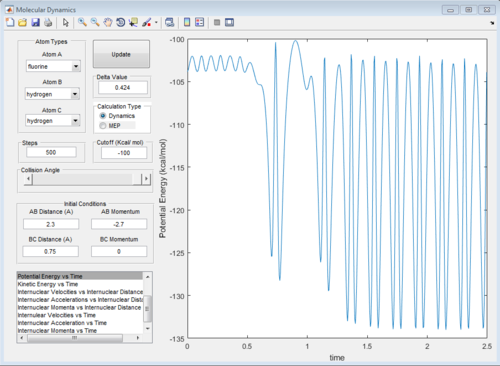 |
The energy in this reaction is converted between kinetic energy (translational energy) and potential energy (vibrational energy) and the total energy of potential and kinetic energy is a constant because of conservation of energy.
At beginning, the changes of kinetic and potential energies are small because it is only due to the vibration of H2 molecule. The potential energy decreases significantly when F atom is close to H2 molecule. The increasing of potential energy at 1.5 s also support the mechanism discussed above. That means F atom move back a little bit.
The reason why the potential energy will decrease is that this reaction is exothermic and the potential energy of reactant is higher than that of product.
This can be conformed experimentally by two ways:
1. IR spectroscopy
The wavenumber of H-F vibration and H-H vibration could be tested by IR spectroscopy and the heat released is stored in the system as potential (vibrational) energy. The vibration energies of them could be calculated by:
E=hv
,where h is plank constant and v is vibration frequency.
2. Thermometer
Energy is released in this reaction; therefore the heat energy changed could be calculated by:
Q = mcΔT
,where Q is the thermal energy absorbed or released, m is the mass of liquid, c is specific capacity and ΔT is change in temperature.
In this part, the reactivity is tested by changing pHH. The reaction condition is:
rHF= 2.30 Å, pHF= -0.5 Ns
rHH= 0.74 Å
The results are showed in Table 2.
The activation barrier of this reaction is very small; so in theory, all of the setup should be reactive. However, none of them are reactive. In conclusion, only momentum AB and momentum BC are at certain value, the reaction will happen.
Nf710 (talk) 16:14, 26 May 2017 (BST) The decaying vincrations willl be lost as heat and hence calorimitry can be used.
Reaction Efficiency
The reaction efficiency could be explained by Polanyi's Empirical Rule[6], which states in a triatomic system, whether vibrational or translational energy is more effective to promote the reaction depends on the position of transition state.
If it is an exothermic reaction, the activation barrier is small which means that the energy of transition state is more similar with the energy of reactants and this also called earl barrier. Polanyi's Empirical Rule claims that for earl barrier, translational energy will promote reaction more than vibrational energy.
For endothermic reaction, the activation barrier is large and the energy of transition state is more close to reactants. This will be called late barrier and in this case, vibrational energy is more effective to promote the reaction.
H atom react with HF is an endothermic reaction. In other word, it is late barrier and vibrational energy should be more effective to promote the reaction. Therefore, the momentum of H-F should be much larger than that of H-H atom. Table 3. showed different pairs of momenta which will make H and HF reaction happen with rHH=2.30 Å and rHF=0.74 Å and this support Polanyi's Empericial Rule.
Conclusion
For H and H2 system, the transition state is at rts = 0.908 Å. Classical Transition State Theory (TST) states that for any reaction the transition state can only pass through once and will never be recrossed. However, the experiment of H and H2 molecule proves that the transition state could be recrossed if the energy is very high.
The system of F-H-H have two possible reactions. The first one is that F atom reacts with H2 and this is an exothermic reaction. The second one is H atom reacts with HF molecule and it is endothermic. Second reaction could be considered as the reversible reaction of the first reaction. Besides, for reaction of F and H2 molecule and reaction of H and HF molecule, they testifies the Polanyi's Empirical Rule which states that for endothermic reaction (late barrier), vibrational energy is more effective to promote reaction and translational energy will promote reaction more for exothermic reaction(earl barrier).
Nf710 (talk) 16:16, 26 May 2017 (BST) Well done for proving the rules
Reference
- ↑ Roussel, M. R. (2009). Transition state theory, 1–5.
- ↑ Roussel, M. R. (2009). Transition state theory, 1–5.
- ↑ Roussel, M. R. (2009). Transition state theory, 1–5.
- ↑ Roussel, M. R. (2009). Transition state theory, 1–5.
- ↑ Meany, J. E., Minderhout, V., & Pocker, Y. (2001). Application of Hammond’s Postulate. An Activity for Guided Discovery Learning in Organic Chemistry. Journal of Chemical Education, 78(2), 204. http://doi.org/10.1021/ed078p204
- ↑ Jiang, B., & Guo, H. (2013). Relative efficacy of vibrational vs. translational excitation in promoting atom-diatom reactivity: Rigorous examination of Polanyi’s rules and proposition of sudden vector projection (SVP) model. Journal of Chemical Physics, 138(23). http://doi.org/10.1063/1.4810007