Ggr07Module2:version1
Analysing Optimised Structures
BH3 Structure
The BH3 structure was optimised for the lowest energy conformation. Optimising the gradient reaches a plateau in which a minimum in total energy is reached at which the gradient apporaches zero. Confirming this is the graphs below at which point the values of bond angle and bond length are recorded (at step number 4). The summary of parameters implemented for calculation are here[4].
| BH3 | BH3 Opt | |
| Bond angle (deg) | 120 | 120 |
| Bond length (Åm) | 1.5 | 1.19 |
BH3 Visualisation of MO's
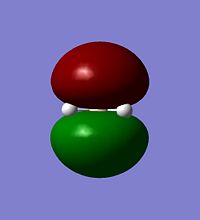
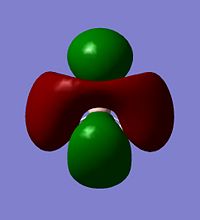
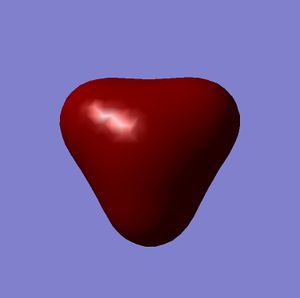
All gaussian calculations here are calculated using Density Functional Theory (DFT) and thus Gaussview can graphicaly represent the output files electron density of the Molecular Orbitals. Some of the orbitals of BH3 around the HOMO are represnted below.
| HOMO-1 | HOMO | LUMO | LUMO+1 |
|---|---|---|---|
 |
 |
 |
|
Calculations of MO's of optimised geometry BH3[5]
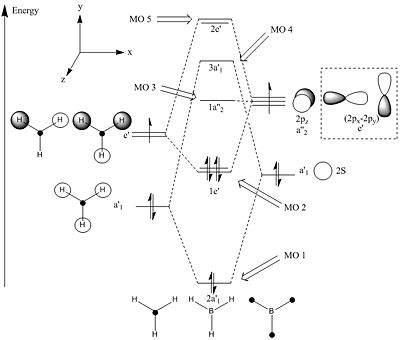
Interestingly with very simple molecules such as BH3 the LCAO is a very accurate representation of the Molecular orbitals comparative to the MO's calculation. The electron density and spin is consistent with the calculated MO's. One of the key downfalls of the LCAO method is that the energy of the MO's can not be calculated and therefore they are only assigned based on intuition and in this example the formation of the diagram can not predict whether the MO of 4 or 5 is highest in energy. The MO calculations predict that MO's 5 are lower in energy than MO 4 and therefore the MO diagram prediction is incorrect. However it is worth noting that this very simple LCAO can give a very quick qualitative view of the MO's without complex mathematics and can help human perspective of the origin of these MO's.
| MO # | Pictorial LCAO | Calculated MO's |
|---|---|---|
| MO 1 |  |
 |
| MO 2 |  |
  |
| MO 3 | 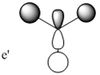 |
 |
| MO # | Pictorial LCAO | Calculated MO's |
|---|---|---|
| MO 4 | 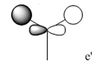 |
 |
| MO 5 |  |
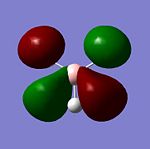 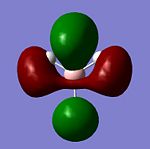 |
BH3 NBO Charges
The natural bond orbital charges can be thought of in a pictorial sense as the "lewis character" of both atoms and therefore the Natural Bond Orbital is the amount of electron density localised around an atom in the MO's relative to the atoms AO's. The pictorial representations of the NBO calculations[6] is shown below.
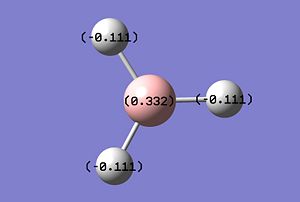
It is worth observing that a negative NBO correlates to an increase in electron density and vice versa. In this example the -0.111 of the protons shows an increase in electron density by -0.111 and the Boron decreased by 0.332. From the calculated NBO there is a large amount of data not represented in the pictorial view can be analised including
- The character of orbitals with respect to hybridisation of atoms as a percentage (sp2 for Boron)
- Interaction between MO's (which isn't significant in this compound Energy of interactions~1.51kcal/mol)
- And the energy and occupation of bonds within a molecule.
In this BH3 example the values of all B-H bonds are tabulated below.
| Molecular Unit | Occupancy | Energy (ckal/mol) | S Character | P Character |
| B-H bond x3 | 1.99853 | -0.43712 | 33.33% | 66.67% |
| B-H Antibond x3 | 0.00147 | -0.41201 | 33.33% | 66.67% |
| Boron 1S Orbital | 1.99903 | -6.64476 | 100% | 0% |
| H atoms Rydberg Orbitals x3 | 0.00032 | 0.90016 | 100% | 0% |
An insignificant amount of occupancy is observed in the B-H antibond. Also an even smaller contribution is observed from the Boron's 1S orbital donated to a vacant rydberg orbital of the three H's. It is also worth noting that the unoccupied orbital of the Boron is represented as S=100% character which is a sign of the inaccuracy of the basis set as it should be an empty P orbital of Boron. The basis set used therefore estimated that the 3S orbital was of lower energy than the 2P resulting in such observation. Therefore an improved larger basis set would be required to return P=100%.
BH3 Frequency
The frequency stretches of BH3 were calculated[7] and found to be of the same energy as the optimised output, this confirms that the optimised geometry has been retained after frequency calculations. It can also be corroborated that the minimum energy conformation is reached by interpreting the lower frequency stretches. For non-linear compounds there should be six and as these values are close to zero (below 10kcal/mol) the ground-state has been confirmed and therefore the minimum energy conformation is reached.
It is worth noting that the displacement vectors pictured were deliberately not scaled up. This was in order to state the insignificance of some parts of the frequency stretch relative to other atoms displacement vectors. It is observed that there is two pairs of doubly degenerate stretches.
A graph plot of frequency against intensity can be made as a prediction of BH3 IR spectrum.
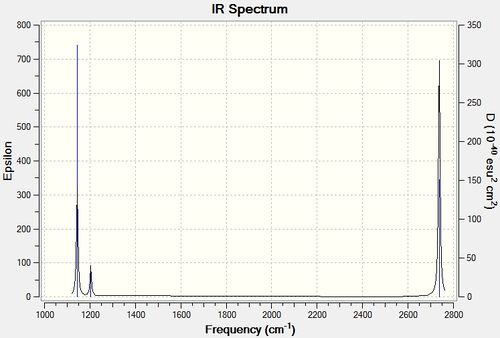
It is observed that there is only two large IR peaks in the spectrum and the large low frequency peak is due to the low frequency single A1 stretch and the other being the higher frequency degenerate pair. The totaly symmetrical stretch is not observed and the other (lower frequency) degenerate pair are of little intensity. These intensities are explained due to the relative dipole change at every frequency stretch with the totaly symmetrical stretch being of zero intensity and the large intesities due to the greatest change in dipole.
BCl3 Structure
The bond length and the bond angle were calculated for BCl3 using the following method[8] which is the same method of Density Functional Theory and also B3LYP method of calculations which is a reputable calculation method for scientific journals. JOURNAL. A slightly larger basis set than BH3 was used being LanL2MB as it includes pseudo potentials for heavier atoms such as Chlorine. It is important to retain the same basis set for both optimisation and frequency analysis. Using a larger basis set in the frequency analysis would be interpreted as being in an excited state relative to the lower basis set/energy of the optimised geometry. As described in the BH3 Frequency section the use of Frequency Analysis not only aids in the understanding of IR spectroscopy but further corroborate that a minimum energy is reached and how accurate it is based on the 6 low frequency stretches and their closeness to zero.
| BCl3 | BCl3 Opt | |
| Bond angle (deg) | 120 | 120 |
| Bond length (Åm) | 1.87 | 1.87 (based on accuray of 0.01Å of 1.86592) |
It is observed that the bond angle is maintained therefore retaining the sp2 hybdrisation. The bond length is observed to be significantly larger due to the Chlorine atom radius being a lot larger than the hydrogen atom. The angle correlates well with values of 1200 (to 30 accuracy) but the bond length not as much with the literature value of 1.72Å. [1] The reason could be due to the fact that the basis set chosen was not large enough to obtain the bond length accurately enough. It was found during frequency analysis[9] that the values of the very low frequency stretches where all below the modulus of 10cm-1 being -8.7725, -4.0872, -4.0872, -0.0021, 0.0050, 0.0093. It must be appreciated that some values are close to the 10cm-1 and therefore accounts for the inaccuracy in the bond angle. The improved larger basis set of LAN2LMB used for BCl3 compared to the basis set of 3-21g of BH3 is confirmed as the Boron empty P orbital is now returned as having P=100% character compared to the S=100% for BCl3
Bonds can be represented in gaussview differently to where they are expected by the viewer. As calculations are made based on the wavefunctions of each atom the bonds are mere formalism during calculations while the overlap of electron density is computed by applying DFT calculations and therefore bonds only indicate which atoms should be close to each other.
Chemical bonds are defined as being purely covalent bond (in which two electrons are shared equaly between two atoms), ionic bonds in which an electron pair is purely localized at one atom and giving net coulombic interaction of a positively charged ion and a negatively charged ion or in the majority of cases an intermediate of these extremes. However this method of interpreting a chemical bond is very much a formalism for humans to be able to pictorialy interpret bonds. A less primitive method of describing a bond would be a shared electron density between two atoms in which all electrons with the correct geometry can contribute to some extent to a chemical bond and the electron density is defined as a probability of an electron being at a positon relative to the atoms.
The symmetry of the BCl3 structure was previously calculated[10] and given the symmetry label of D3h which are compounds which have the following properties 2C3, 3C'2, σh 2S3, 3σv which is expected of this molecule. The opposite method of thinking of this is that if these properties are imposed on the molecule there is constraints as to the possible geometry and therefore by limiting possible geometries there are less iterations required to reach a possible energy minimum therefore reducing calculation times down to 19s.
Isomers of Mo(CO)4L2
The isomers of Mo(CO) cis and trans differ in the positions of the PPh3 and this effects some properties of the complex as will be explored below.
Optimisation of Structure of Cis and trans Mo(CO)4(PCl3)2
PCl3 has been recorded (journal) to be a very good approximate in calculations as to the inductive properties of PPh3. Due to the timescale of the project PCl3 groups will be modeled for calculations as this group drasticaly decreases the amount of non hydrogen atoms to be computed in calculations therefore reducing calculation times.
The cis and trans Mo(CO)4</sub.>(PCl3)2's geometry were first optimised using B3LYP calculation method and a low level basis set and pseudo-potential LANL2MB in order to get a rough conformation. It was observed that the both isomers energies converged (i.e reached a minima).
Resulting geometries were then altered by rotating the PCl3 groups to a higher energy in which the minima of lowest energy would be found during the following optimisation.
The cis and trans were then further optimised using a larger basis set of LANL2DZ with a tighter convergence limit to reach a better optimisation of geometry. The resulting Cis[11] and Trans[12] were found to converge in energy.
The improved optimisation can be further optimised as the Phosphorus low lying d Atomic Orbitals have not been accounted for even in this basis set. It is known that phosphorus compounds are often commonly observed with phosphorus in the hypervalent state. Therefore a further optimisation can be made with these d orbitals and the resulting Cis[13] and Trans[14] opimisation were calculated with convergence of the Energy.
Frequency Analysis of Cis and trans Mo(CO)4(PCl3)2
Frequency Calculations were made using the same basis set of LANL2DZ (with Phosphorus d orbtials) for the cis and trans isomer with the resulting Force converging but not the Displacement. However further analysis of the cis[15] and trans[16] frequency output files confirmed that the optimisations and the frequency calculations had been succsesful due to the isomers respective 6 low frequencies being <10kcal/mol (and negative values ~ 0 kcal/mol).
Structural Commentry
| Bond | Reference [2][3] | Cis | Difference |
| Mo-C | 2.063 | 2.035 | -0.028 |
| C-O | 1.1283 | 1.175 | 0.0467 |
| Mo-P | 2.52 | 2.58 | 0.06 |
| P-Cl | 2.039 | 2.12 | 0.076 |
| Bond | Reference [2] [3] | Trans | Difference |
| Mo-C | 2.063 | 2.057 | -0.006 |
| C-O | 1.1283 | 1.17 | 0.0417 |
| Mo-P | 2.52 | 2.42 | -0.1 |
| P-Cl | 2.039 | 2.12 | 0.081 |
Energy Commentary
It is known that a complex stability depends on the strength of the bonds to the ligands. Considering the σ interactions only, will not give an accurate picture as π backbonding and donation is known to occur also between the d-orbitals of the metal and the ligand. The more backbonding that is apparent (combined with the lewis basicity of the ligand) the more stable the complex.
Both carbonyl and trichlorophosphine are π acceptors. The carbonyl ligand due to it's π*C-O and attract electron density which strengthens the Mo-C bond and weakens the C-O bond. The phosphine also has an empty MO which is correctly aligned on the phosphorus to π accept.
Therefore there will only be a marginal difference in energy between the cis and trans as the chlorine substituents will increase the inductive effect on the phosphorus increasing it's π accepting ability. However the carbonyl is expected to be a slightly better π acceptor.
Knowing this the cis isomer is expected to be slightly more stable due to its ability to accept more electron density from the electron rich molybdenum as two carbonyls are competing with phosphines for electron density while the trans carbonyls are merely competing against each other for electron density.
| Isomer | Energy | Units |
| Cis | -623.577072 | hartree |
| Trans | -623.576031 | hartree |
| ΔE | -0.00104093 | hartree |
| ΔE | -2.73296172 | kJ/mol |
| Isomer | Energy | Units |
| Cis | -623.692912 | Hartree |
| Trans | -623.694156 | Hartree |
| ΔE | 0.001244 | Hartree |
| ΔE | 3.266122 | kj/mol |
The 2nd optimisation shows that the cis is more stable than the trans whereas the 3rd optimisation (which includes the low lying d-orbitals of phosphine) says otherwise. The key point is that the energy difference is marginal between the two isomers as it is important to appreciate that the energies are only accurate to the nearest 0.004hartree (~10kJ/mol). Even though it is anticipated that experimentaly the cis isomer will be the most stable the difference is quite marginal and therefore altering the ligands from this almost "centre point" can favour one isomer over the other.
If the trans isomer wanted to be favoured (lowest energy) steric bulk could reduce the stability of the cis and make the two phosphine groups become trans to each other in order to form a stable compound. Therefore the original triphenylphosphine could be used as it's inductive effect would be similar but it's steric bulk would be far larger.
If the cis isomer wanted to be favoured the ability of the phosphine to accept electron density could be decreased by putting less electronegative substituents on the phosphine such as PPH3.
IR Commentary
It was observed that there were two very low energy frequencies apparent in both the cis and trans isomers. These mainly involved rotation around the σMo-P bond of the trichlorophosphines and due to the geometry of the trans isomer, rotation also by the four carbonyls around the Mo.
As specified earlier each isomer had two lower frequencies and these arise as both PPCl3 groups can rotate clockwise/anticlockwise or one rotate clockwise and the other anticlockwise.
At room temperature (knowing boltzmanns distribution) these low frequencies are highly accesible and therefore it would be expected that at room temperature these accesible rotations would cause the PPCl3 to whizz around in circles.
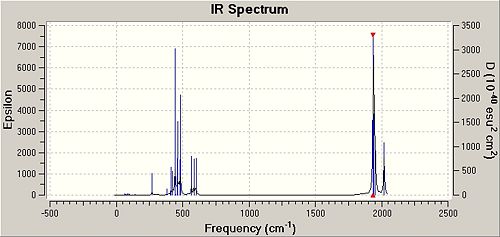
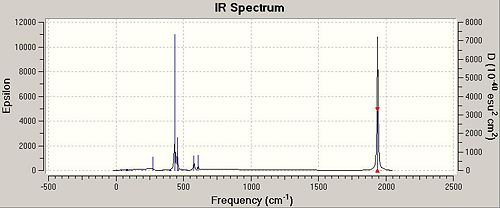
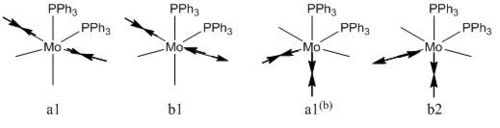
It is important to realise that symmetrical stretches are IR inactive (as a dipole is not created) and therefore would not appear in an IR spectra (these stretches are apparent as close to 0 intensity in the frequency list).
In the cis isomer there are two carbonyls trans to a PPh3 and two trans to each other therefore the pairs will be excited at different energies. Both a1 and a1(b) would be inactive for the cis resulting in two IR stretches b1 and b2. b1 is the intense peak and b2 is the weak peak as dipole change is most significant in b1

All carbonyls are in the same environment in the trans complex. Therefore it is expected that all carbonyls are to be excited at a given energy. There are four possible vibrations but only the Eu doubly degenerate is IR active therefore we expect to see one intense peak.
Mini project
Background
Project Based on the journal - Boryllithium: Isolation, Characterization, and Reactivity as a Boryl Anion, Yasutomo Segawa, et al.; Science 314, 113 (2006); DOI: 10.1126/science.1131914
Boron is an element which predominantly exists as an electron poor species in compounds such as the BH3 and is known for its character as a lewis base due to its lone pair. Boron (and Aluminium) compounds which are electron rich do exists to the extent of being almost negatively charge. NaBH4 (and LiAlH4) is an example of a negatively charged species and is used extensively in organic chemistry.
However there has been much research in expanding group 13 chemistry by reversing its natural chemistry. Examples of Boron behaving as a nucleophile have proven elusive. The focus of research has been on an attempt to form a carbene analogy in which the boron has close to a full negative charge.
In 2006 Yasutomo Segawa, et al. successfully synthesised the first Boron nucleophilic compound. It was a NHC (Nitrogen Heterocycle Carbene) analogous boryl anion which was synthesised and then its chemistry observed with a range of electrophiles.
What is to be studied?
The purpose of this experiment is to explore this new chemistry and hypothesise future structures and potential for new group 13 nucleophiles. The first half of the project is based on the Boron structure and the second half expands to other group 13 elements.
The 2,6-dips (2,6-diisopropylphenyls) which are bound to the Nitrogens are strategically added to increase the stability of the boryl anion as the steric bulk of these groups protects the compound from dimerising and become unreactive as nucleophiles,
Due to the short duration of the project the study of the di-isopropylphenyl groups are not essential for this study as the steric interaction is not the focus of the study and therefore they have been replace by chlorine atoms which have been regularly used as substitutes for phenyl groups. It is appreciated that the electron withdrawing ability of the dip groups will be slightly less due to the isopropyl groups increasing electron density in the phenyl rings.
The Structure
The Borons are both part of a planar 5 membered ring in the boryl and boryl anion. The B-H (hydrogen bound Boron) is in its natural polarity and is stable and obeys Huckels rules (as both Nitrogens and the alkene contribute 2 electrons each obeying the Huckel 4n+2=6). The B-Li (Lithium bound Boron) retains this aromaticity due to the empty p orbital giving extra stability explaining the success in the synthesis of this carbene analogy.
The B-H and B-Li compounds geometry were both optimised using MM2 followed by DFT using B3LYP calculations with LANL2MB as a basis set. This optimised geometry was then optimised with a better basis set of LANL2DZ. A frequency analysis was made to check that a minima had been reached by checking for the appearance of 6 low frequencies >10cm-1. Key points comparing the geometry and properties are tabulated below.
| NBO of B-H | NBO of B-Li |
|---|---|
 |
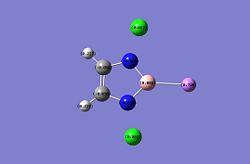
|
| Properties | B-H | B-Li |
| B-H/Li bond length (Å) | 1.17 | 2.13 |
| B-N bond length (Å) | 1.44 | 1.47 |
| N-B-N Bond Angle (deg) | 100.6 | 95.9 |
It is observed from the charge distribution using NBO that the Boron is far more electropositive in the B-H rather than the B-Li this is expected as the B-H is expected to be more covalent while the B-Li is observed as a Boron anion. Even so it is worth noting that the natural bond order does not become negative although very close to zero. This might be due to the inaccuracy of calculations or that the negative charge is well withdrawn by the aromatic ring therefore the lone pair is on the Boron which is likely as the NBO of the lithium is very positive 0.725 almost 1+ oxidation state +1. To furthur corroborate the B-N bond length is longer and N-B-N bond angle are narrower in the B-Li which is expected due to repulsion of negative charge from the B and corroborates with the observed geometry in the original literature. The bond length of the B-Li is expected to be larger than the B-H due to the larger size of the atomic radius of the lithium.
Electron Density in the Aromatic Ring
The purpose of this study is to observe if altering the electron density can affect the stability of the boryl anion with the possibility of enhancing its nucleophilicity increasing its chemistry even further. It is possible to alter the substituents attached to the alkene and Nitrogens in order to alter the electron density. It is important however to appreciate that alterations to the Nitrogen substituent’s would in reality need to be applied to the dip groups and therefore further calculations with substituent’s on dip would be need to get accurate result. Only a trend is looked for in this study.
It is predicted that the anionic charge on the Boron can be stabilised further by adding EWGs (Electron Withdrawing groups) groups to the ring which will increase the ionic character of the B-Li bond, the reverse can be done by adding EDGs (Electron Donating Groups).
The EDG chosen is OMe as the oxygen resonance of its lone pair makes it highly donating. The EWG chosen is a CN group which can resonate away from the ring. The electron density can further be withdrawn by exchanging the Chlorines for Fluorines (which are more electronegative) named EWG+.
It was realised due to the method of MM2 it does not consider the wavefunctions of the atoms and therefore doesn’t consider the stability of the aromaticity therefore it is essentially a step back in terms of optimisation therefore the optimisation of geometry was initiated with DFT LANL2MB and then improved with LANL2DZ. A frequency analysis was made to check that a minima had been reached by checking for the appearance of 6 low frequencies >10cm-1. The doubly optimised compounds were compared below in terms of the mulikhen charge distribution , NBO, bond angles and MO’s.
| Compound | N-B-N Bond Angle (deg) | B-N bond length (Å) | B-Li bond length (Å) | Boron NBO Charge |
| EDG | 97 | 1.46 | 2.13 | 0.094 |
| B-Li | 95.9 | 1.47 | 2.13 | 0.088 |
| EWG | 96.6 | 1.47 | 2.14 | 0.124 |
| EWG+ | 94.6 | 1.45 | 2.13 | 0.033 |

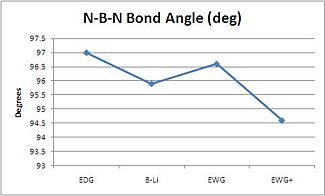
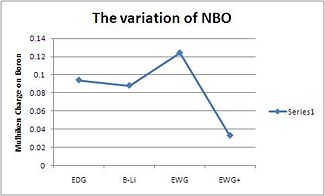
Most of the data seem to correlate quite well. The N-B-N bond angle decreases as the Boron becomes more negatively charged which directly correlates with the NBO's. However the EWG group seems to be an anomaly it's bond angle has increased and the NBO has dramatically increased. This could be due to the fine balance of aromaticity and ionic character of the boron. As the boron gains more electron denisty the N-B-N angle narrows but as the aromaticity is also decreased the N-B-N widens. It seems apparent that the alkene CN substituents withdraws electron density from the alkene dramaticaly and the N-B-N less (due to the reverse of sign of the alkene carbons NBO compared to H as substituents). The B-N bond lengths seems to vary very little (2pm) during these transitions.
Expanding to other Group 13 Elements
The presence of the electronegative Boron was proven in the previous section and therefore it would be interesting to study whether more electropositive elements of this group could be turned in to nucleophiles.
The outcome is unpredicted and will be intriguing to study. On the one hand this type of compound has been able to give unnatural polarity to the Boron but the electropositivity of Al and Ga might be too much to be compensated for therefore making the Lithium bond more covalently bound and not a carbene analogy.
The EWG+ will be used for comparison. The Boron is switched for Al and Ga and optimised using DFT the structures were directly optimised with LANL2DZ basis set but due to the presence of heavier atoms B, Al and Ga were further optimised with an extrabasis set for their low lying d-atomic orbitals. A frequency analysis was made to check that a minimum had been reached by checking for the appearance of 6 low frequencies >10cm-1. The doubly optimised compounds were compared below in terms of the mulikhen charge distribution, NBO, bond angles and MO’s.
| Compound | N-M-N Bond Angle (deg) | M-N bond length (Å) | M-Li bond length (Å) | Boron NBO |
| B-EWG+ | 94.6 | 1.45 | 2.13 | 0.033 |
| Al-EWG+ | 79.1 | 1.87 | 2.59 | 0.712 |
| Ga-EWG+ | 77.8 | 1.91 | 2.53 | 0.606 |
It is worth noting that the bond lengths can not be directly compared due to the difference in atomic orbital size. The NBO's do represent the relative electronegativities described in Pauli's/Muliken's scale in which the Aluminium is most electropositive followed by Gallium and finaly Boron. It seems due to the strong electropositivity of Gallium and Aluminium that this structure is not suitable for forming Aluminium and Galliium nucleophiles and that drastic adaption or a new structure needs to be devised.
Extra attempt at Al
Due to the inability to reduce the NBO of Al, a further attempt was made by changing the flourines on the nitrogens. Peptide bonds are known to be very stable and the carbonyl group is very electron withdrawing. By substituting the protons on a methyl group for fluorines a moiety analogous to TFA (Trifluoroacetic Acid) can be added. TFA is known to have a pKa of 0.3 JOURNAL which shows its large electron withdrawing ability. By substituting the two fluorines gives a far greater opportunity for reversing the polarity. B3LYP calculations were used and LANL2DZ basis set was implemented then further optimised with an extrabasis set for their low lying d-atomic orbitals. A frequency analysis was made to check that a minimum had been reached by checking for the appearance of 6 low frequencies >10cm-1.
| Compound | N-M-N Bond Angle (deg) | M-N bond length (Å) | M-Li bond length (Å) | Boron NBO | Lithium NBO |
| Al-EWG+ | 79.1 | 1.87 | 2.59 | 0.712 | 0.662 |
| Al-EWG++ | 82.7 | 2.01 | 2.68 | 0.877 | 0.735 |
It is interesting that the contradictory is observed to the first assumption that the Aluminium bond order would reduce the N-M-N bond angle would decrease and the bond length would increase. The M-Li bond length is significantly larger than all previous molecules. This is due to the conformation of the molecule. The Lithium NBO was added to show that the bond length is longer but also the NBO of Lithium has become more positive i.e. leads to the understanding that the electron pair has been donated fully to the Boron.

From visualisation of the molecule it can be seen that the Lithium is far closer to the Oxygen which would further indicate that the boryl anion has been formed. Ironically by forming a more stable "Borene" it's reactivity as a nucleophile might have been diminished by a more predominant resonance of the negative charge on the oxygen in which the lithium is more closely chelating to it and therefore the compound will more likely act as a nucleophile at the oxygen.
Summary
It has been possible to model the Boron species and all anomalies have been attempted to be interpreted. Even after exhaustive attempts it has been unsuccesful in making other group thirteen analogies in to nucleophiles.
Calculations
Calculations made during the mini-project are references below. Only final optimisations are displayed as they are only deemed neccesary.
08/12/09 - Currently there is an error in publishing in to D-space on my user explaining the empty spaces I am awaiting for this issue to be resolved by ICT and then they will be published.
10/12/09 - Issue has been resolved and all relevant calculations have been published in D-space
References
- ↑ [1], Henri A. Lévy, L. O. Brockway, "The Molecular Structures of Boron Trimethyl, Trifluoride, Trichloride, and Tribromide.", J. Am. Chem. Soc., 1937, 59 (11), pp 2085–2092, DOI: 10.1021/ja01290a002.
- ↑ 2.0 2.1 [2], IV*, Steric Influences on the chelation of ditertiary phosphine complexes of group VI metal carbonyls, J.A. CONNOR and P.I. RILEY Journal of Organometallic Chemistry 94 (1975) 55-60 doi:10.1016/S0022-328X(00)82529-2.
- ↑ 3.0 3.1 [3], CRC Handbook of Chemistry and Physics 2009-2010, section 9 pp27,34 and 42.