Further Abstract & Syntheses
Abstract
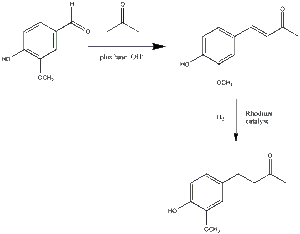
Zingerone takes the systematic name of (4-(4-hydroxy-3-methoxyphenyl)-2-butanone). As mentioned, it is the natural flavour that is a derivative of the even more basically occurring zingerol. There are several methods by which it can be produced, and not just by the simple process of 'cooking'. Below is a schematic of the path by which it can be synthesized by cross-aldol condensation, followed by a catalysed hydrogenation.
Zingerone Alternative Synthesis
Derivatives
As can be observed from the image as above, there is a methoxy group para to the hydroxy group on the phenyl ring. If this group were to be replaced by a hydrogen atom, it would be converted to rheosmin (otherwise known as the 'raspberry ketone'), another naturally occurring flavour. However, for this, the starting reagent can not, obviously, be vanillin, so instead 4-hydroxybenzaldehyde is used, which is essentially a direct analogue of the rheosmin (as zingerone is to vanillin). Further to this, small amounts of zingerone can be found in raspberries, as well as in the naturally greater amounts in ginger roots.
Other Properties
As would be expected, zingerone occurs as crystals that can vary in colour from dirty yellow to faded brown. Strangely, though, with its numbers of sites of electronegativity (three oxygens spaced out across the compound), one would have assumed that since the structure was more long than large that it would be in the liquid phase at room temperature. As below, we note that it is solid, but it still liquidates at a relatively low temperature. Instead, the aforementioned property affords itself to the high boiling point. However, it would be fair to note that the impedement of tail prevents the full effect of the dipoles from taking place, hence why zingerone has a boiling point that is barely higher than that of vanillin (285°C).
| Physical & Other Properties | ||
| Melting Point | 40-41°C at 1 atm | |
| Boiling Point | 290°C at 1 atm | |
| Refractive Index | 1.544 at 20°C | |
| Natural Concentration | 80.00 ppm | |
| Water Solubility | Insoluble | |
| Organic Solubility | to 1.1% in ethanol | |
History & Background
It would be fair to note that zingerone (Zingiber officinale(and thus its derivative of zingerol) is part of a set of compounds known as the vallinoids (which includes the following: vanillin (Vanilla planifolia); capsaicin (chile pepper: Capsicum annuum); and eugenol (clove: Eugenia caryophyllis). All of these compounds have the characterisation of having a specific set of receptors on sensory cells in the relevant areas of the central and peripheral nervous systems. Each compound binds to a particular receptor, thus causing a fluctuation in the level of sensory effects. Of course, each of the vallinoids has a differing (quantitative and qualitative effect). Zingerone, relatively speaking is quite neutral in its effect, though it will dull certain sweetnesses, and offers moderate change to the said senses.
References
Extended thanks to the following locations and parties for the use of their material:
Rheosmin (“Raspberry Ketone”) and Zingerone, and Their Preparation by Crossed Aldol-Catalytic Hydrogenation Sequences The Chemical Educator: Chemistry & Materials Science (Vol. 1, No. 3, August 1996) Leverett R Smith
Vanilla & Other Medicinal Spices & Their Effects The University of California: Los Angeles, History and Special Collections: the Louise M. Darling Biomedical Library (Online)
Zingerone: Properties (Physical & Otherwise) The Good Scent Company: Scents & Chemical Database
