Ephedrine
| Ephedrine | |
|---|---|
| |
| IUPAC Systematic name | |
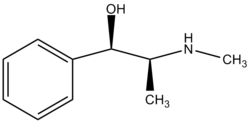
| (1R,2S)-2-(methylamino)-1-phenylpropan-1-ol | |
| Other name | |
| (αR)-α-[(1S)-1-(methylamino)ethyl]benzenemethanol, α-[1-(methylamino)ethyl]benzyl alcohol, or L-erythro-2-(methylamino)-1-phenylpropan-1-ol | |
| Indentifiers | |
| CAS number | 299-42-3 |
| PubChem (CID) | 5032 |
| SMILES | [C@@H(C)[C@H](O)C1=CC=CC=C1 |
| Chemical Data | |
| Molecular formula | C10H15NO |
| Molar mass | 165.23 g/mol |
| Pharmacokinetic Data | |
| Bioavailability | 85% |
| Metabolism | minimal hepatic |
| Half life | 3-6 hours |
| Excretion | 22-99% renal |
| Therapeutic considerations | |
| Pregnancy cat. | A |
| Legal status | Prescription only |
| Routes | Oral |
Introduction
Ephedrine is a sympathomimetic amine with a structure similar to the synthetic derivatives amphetamine and methamphetamine. Ephedrine is commonly used as a stimulant, concentration aid, to help weight loss and to treat hypotension. Chemically, it is an alkaloid derived from various plants in the genus Ephedra (family Ephedraceae). It is most usually marketed in the hydrochloride and sulphate forms.
The 3D Molecular Structure
Hyoscine |
Picture of Ephedrine Tablets

The picture shows the most common form of the drug Ephedrine. It is a small white tablet with a letter on. This form is Ephedrine hydrochloride. Sulphate forms of the tablet are also available.
History of Ephedrine
Ephedrine has been found in traditional Chinese medicines. Examples include the herb ma huang Ephedra sinica) which contains ephedrine and pseudoephedrine.
Nagayoshi Nagai was the first person to isolate ephedrine from Ephedra vulgaris in 1885. The production of ephedrine in China has become a multi-million dollar export industry. Companies producing for export extract US$13 million worth of ephedrine from 30,000 tons of ephedra annually.
Chemistry of Ephedrine
Ephedrine exhibits optical isomerism and has two chiral centres. By convention the enantiomers with opposite stereochemistry around the chiral centres are designated ephedrine, while pseudoephedrine has same stereochemistry around the chiral carbons. That is, (1R,2R)- and (1S,2S)-enantiomers are designated pseudoephedrine; while (1R,2S)- and (1S,2R)-enantiomers are designated ephedrine.
The isomer which is marketed is (-)-(1R,2S)-ephedrine.
Ephedrine may also be referred to as: (αR)-α-[(1S)-1-(methylamino)ethyl]benzenemethanol, α-[1-(methylamino)ethyl]benzyl alcohol, or L-erythro-2-(methylamino)-1-phenylpropan-1-ol. Ephedrine hydrochloride has a melting point of 187-188°C. [1]
As with other phenylethylamines, it is also somewhat chemically similar to methamphetamine, although the amphetamines are more potent and have additional biological effects.
Uses of Ephedrine
Some reports have suggested that Ephedrine helps studying, thinking, or concentrating to a greater extent than caffeine. It is more commonly used by professional athletes and weight lifters as a stimulant. It is common for many athletes to use stimulants while exercising. Many people have started to use Ephedrine as helps burn fat during exercise to aid with weight loss. [2]
Ephedrine also has a structure similar to amphetamines. Ephedrine can be used in the synthesis of methamphetamine by chemical reduction making Ephedrine a highly sought-after chemical precursor in the illicit manufacture of methamphetamine. Ephedrine can be reduced to methamphetamine by using a synthesis similar to the Birch reduction. In the reduction of Ephedrine both anhydrous ammonia and lithium metal are used. The second most popular method uses red phosphorus, iodine, and ephedrine in the reaction.
Through oxidation, ephedrine can be easily synthesized into methcathinone. Ephedrine is listed as a Table I precursor under the United Nations Convention Against Illicit Traffic in Narcotic Drugs and Psychotropic Substances. [3]
Ephedrine is also used by some party goers as a weaker version of the popular but dangerous drug Ecstacy.
Problems with Ephedrine
Like most stimulants, Ephedrine has many side effects. It can cause stomach pains, headaches and dilation of the pupils.
Ephedrine has been reported to cause both physical and psychological dependence after excessive long-term use. This is particularly true with oral forms of ephedrine as used by athletes as it can cause stimulant dependance. Ephedrine has been linked to deaths from heatstroke in athletes and circulatory problems such as aortic aneurysm in weightlifters.
Ephedrine should not be used by people with poor fluid replacement, impaired adrenal function, hypoxia, hypercapnia, acidosis, hypertension, hyperthyroidism, prostatic hypertrophy, diabetes and cardiovascular disease.
Ephedrine should NOT be used at any time during pregnancy.
References
- ↑ Budavari S, editor. The Merck Index: An encyclopedia of chemicals, drugs, and biologicals, 12th edition. Whitehouse Station: Merck
- ↑ http://astronutrition.com/weight-loss-weight-loss-ephedrine-ephedra-25_1/
- ↑ http://www.incb.org/pdf/e/list/red.pdf
