Ejc107:001
Introduction
Imperial College London Computational Chemistry 3rd year Lab using molecular mechanics and semi-empirical molecular orbital methods to evaluate structures and the corresponding spectrums.
Calculations on set problems and a structure based mini project as defined by Mod:organic
Carried out and written by, Elliot Carrington
Module 1
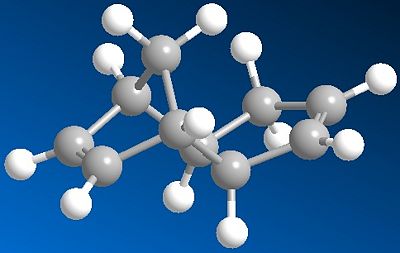
Exercise 1: the Hydrogenation of Cyclopentadiene Dimer
It's been reported that the dimerisation of cyclopentadiene produces specifically the endo product. In order to assess the relative energies and stabilities of the endo and exo product MM2 optimisations were carried out using ChemBio3D.
The modelled Endo, Exo products and their calculated energies are shown below:
From the optimised data obtained the exo product is predicted to be lowest in energy and most thermodynamically stable, The endo product predominating shows that the reaction therefore is under kinetic control.
An interactive Jmol Image of the exo and endo products is available from button below:
Hydrogenation of Cyclopentadiene
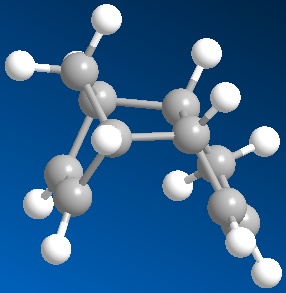
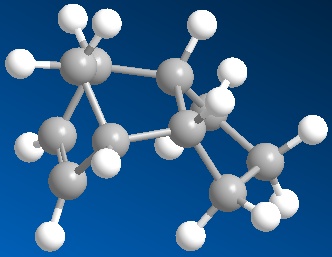
The cyclopentadiene dimer subsequently undergoes hydrogenation at either the 6 carbon ring double bond or 5 carbon ring double bond. MM2 optimisation was again used to access the ease of hydrogenation based on the thermodynamic stabilisation
Shown below is the modelling results:
The overall energy of the hydrogenation product 4 is lower suggesting a more thermodynamic stable structure and that initial hydrogenation of the endo product will result with this double bond being removed first
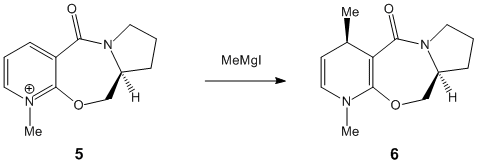
Exercise 2: Stereochemistry of Nucleophilic additions to a pyrindinium ring (Nad+ analogue)
This exercise is concerned firstly with the alkylation in the 4 position of the pyridine ring of an optically active derivative of proliniol to form an specific stereochemical product, and secondly another derivative involved in the nucleophilic transfer of NHPh to the 4 Position of the pyridinium ring.
The reaction scheme is showed below:
Reaction 1
The above image defines the exact stereo chemistry of compound 6. Carrying out optimisation of the molecule gave the other isomer as the most stable and therefore the reaction has to be considered to be under kinetic control where the most stable reaction pathway will occur. Shultz et al report[1] that the alkylation of pydinium ring using a grignard is greatly influenced by the chelation to carbonyl groups such as the one in the optically active prolinol derivative as shown below (taken from lit above):
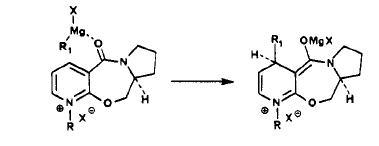
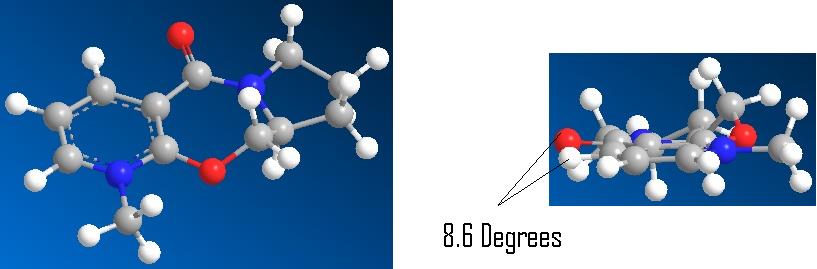
Optimisation of the reactant 5 showed a distinct preference for the Carbonyl to be slighlty orientated above the ring 8.6o, i.e. aligned with the incoming grignard. This is shown in the 3D Image below:
Energy of 57.39Kcal/mol calculated
This confirms the chealation to the carbonyl group, This would not be the same for alternate methods such as alkyl lithium with is reported to give no stereo control
Calculations involved used ChemBio3D and MMFF94 force field
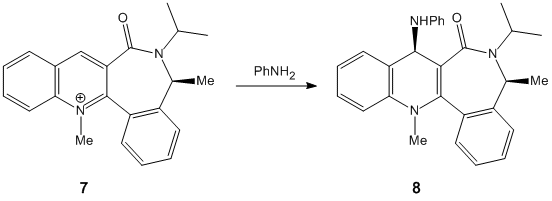
Reaction 2
The second reaction involves a similar analytical approach.
Optimisation again using the MMFF94 Force Field resulted in the molecule shown below (best viewed via the Jmol):
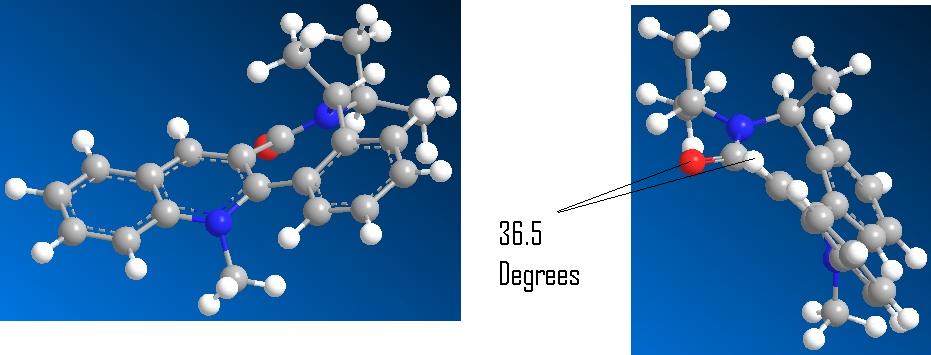
The optimisation revealed the carbonyl below the face relative to the orientation of the methyl group at angle of -36.5. This is consistent with the reported structure by Leleu et al[2].Unlike the above example however the incoming NHPh group ends up on the top face as defined by the stereospecific product while the carbonyl is on the bottom face. This suggests the kinetic driving force is either electronic or steric repulsion from the CO Group rather than chelation
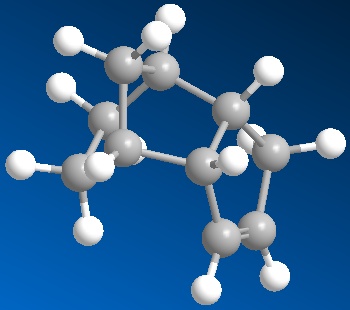
Exercise 3: Stereochemistry and Reactivity of an Intermediate in the Synthesis of Taxol.
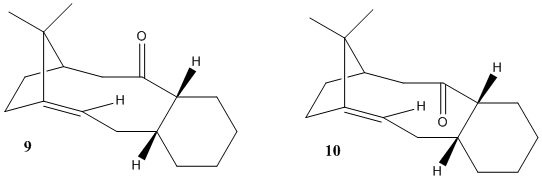
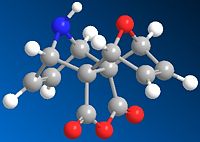
During the synthesis of taxol an interesting intermediate is formed in the two isomers shown below:
The least stable isomer is reported to readily isomerise to the more stable intermediate before further steps are carried out. The isomers are atropisomers and depend on the orientation of the carbonyl group
Minimisation calculations using MM2 ChemBio3D resulted in the following Results:
From the data obtained isomer 10 can be seen to be the more stable, the major difference being in the bending energy
From the 3D Image of isomer 10 (best viewed through Jmol) it can be seen that the double bond is at a bridge head which under bredts rules should be disallowed, However it is reported to be very stable to hydrogenation. McEwen et al[3] report that in some hyperstable olefins with medium sized polycyclic rings that have double bonds at bridgehead can be favoured. This tends to occur when the strain energy of the hydrogenated product exceeds that of the original alkene.
Using this idea the Hydrogenation product was analysed and its energy minimised using the MM2 force field.
| Total Energy: 72.89Kcal/mol | Stretch: 4.69Kcal/mol | Bend: 23.90Kcal/mol | Stretch-Bend: 1.12Kcal/mol | Torsion: 22.04Kcal/mol | Non-1,4 VDW: 3.25Kcal/mol | 1,4 VDW: 17.89Kcal/mol | Dipole/Dipole: 0.00Kcal/mol |
Interestingly it revealed that the energy of the hydrogenation product is much higher particularly for bend energy than the alkene (isomer 10)and therefore can be considered to fall into the hyperstable olefin category.
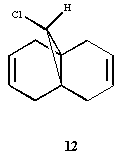
Exercise 4: Regioselective Addition of Dichlorocarbene
Compound 12 shown below exhibits high selectivity to electrophilic reagents i.e. dichlorocarbene.
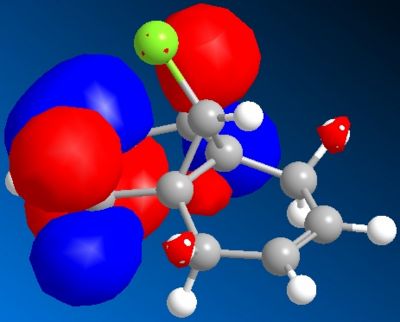
In order to investigate the reasoning for this MOPAC/PM6 was used to give an approximate representation of the valence electron wavefunction focusing on the highest occupied molecular orbital (HOMO).
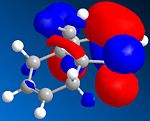
The HOMO of compound 12 is shown below and can be seen to shown high density over the double bond syn to the overhanging chlorine.
This suggests that this double bond is the most nucleophilic and most susceptible to electrophilic attack
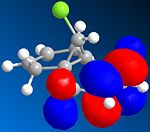
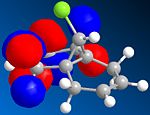
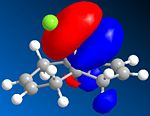
The other molecular orbitals are shown below:
| HOMO -1 | LUMO | LUMO + 1 | LUMO +2 |
|---|---|---|---|
 |
 |
 |
|
| Anti C=C Bonding | Anti C=C antibonding | Syn C=C Bonding | C-Cl Antibonding |
Literature sources[4] suggest that the anti double bond is stabilised by overlap of the C-Cl antibonding (LUMO + 2) with exo (anti)Pi orbital (HOMO -1) resulting in the endo (syn) double bond being more nucleophilic.
This is supported by the molecular model calculated as it can be seen that the anti C=C is oriented more towards the bridgehead than the syn double bond.
The computational model was submitted to scan in order to calculate the vibrational frequencies for the both compound 12 and it's hydrogenated (anti to Cl) version. This is designed to help calculate the influence of the C-Cl bond on the vibrations of the molecule.
Shown below are the infrared spectrums for both compounds and relevant stretches compared to frequency
| Compound 12 | Hydrogenated Compound 12 |
|---|---|
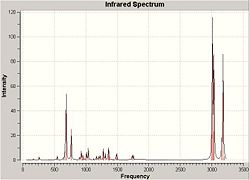 |
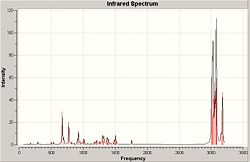
|
| Strech | Frequency | Compound 12 | Frequency | Hydrogenated 12 |
|---|---|---|---|---|
| C-Cl | 770.93 | 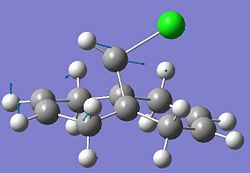 |
774.97 | 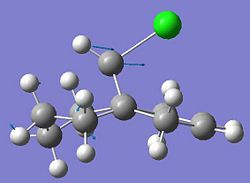
|
| Anti C=C | 1736.99 | 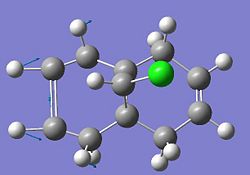 |
N/A | N/A |
| Syn C=C | 1757.32 | 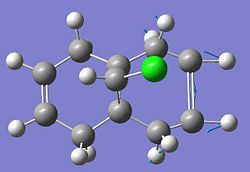 |
1758.05 | 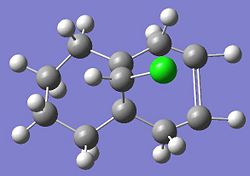
|
| C-H (C=C Anti) | 3178.75 | 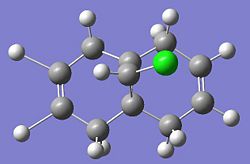 |
N/A | N/A |
| C-H (C-C Anti) | N/A | N/A | 3078.06 | 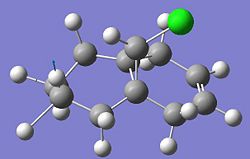
|
The spectrums show high similarity, they both contain high intensity peaks at 3000-3300, 800, 650 cm-1
However the hydrogenated product shows increased stretching frequencies around 3000cm-1 which looking at the key vibrations can be attributed to the increase of C-H orientations after the removal of the double bond. The non-hydrogenated product however contains more stretching modes at 3100-3200cm-1 indicative of C-H stretches for hydrogen's on double bonds.
The stretches of the C=C syn to the C-Cl are very similar to each other for both the hydrogenated and non hydrogenated form. The Stretch for the anti C=C however is significantly lower in frequency and therefore energy. This is likely due to the donation into the C-Cl antibonding orbital weakening the Pi Bond.
Structure based mini project
Cycloaddition reaction of pyrrole with norbornadiene-2,3-dicarboxylic anhydride modified at the 7 position to an Oxygen atom (IUPAC name: 7-Oxabicyclo[2,2,1] hepta-2,5-diene-2,3-dicaboxylic anhydirde) to give selective exo,exo adduct.
This project is based on analysis and comparisons of work carried by Margetic et al[5] and all literature sources are from the original journal
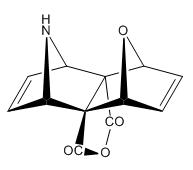
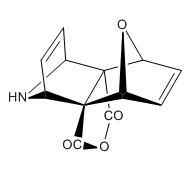
The reaction scheme is shown below and reported to give the stereospeifcic adduct with the two hetrobridges syn orientated:
The alternate isomer possible is the exo,endo adduct with is only reported for very bulky nitrogen substituent's such as N-Trityl.
The two possible isomers of the pyrrole attack are shown below:
(N.B. The exo,exo and exo,endo notation is taken from the Lit Source)
| Syn Orientated | Turn Frame Adduct |
|---|---|
 |
|
 |
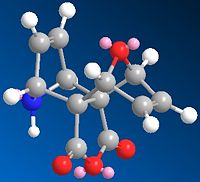
|
| MM2 energy: 79.73 Kcal/mol | MM2 Energy 82.85 Kcal/mol |
The two isomers are very close in energy however the exo,exo adduct seems to thermodynamically more stable although the reaction is likely to be under kinetic control and dependent more on the transition energy barriers of the various orientations
The two reactants were modelled and are shown below:
| Pyrrole | O-bridged Norborndiene-2,3 dicarboxylic anhydride |
|---|---|
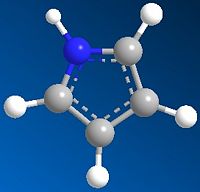 |
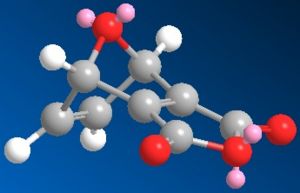
|
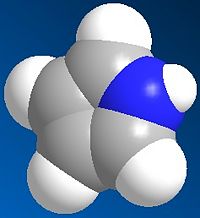 |
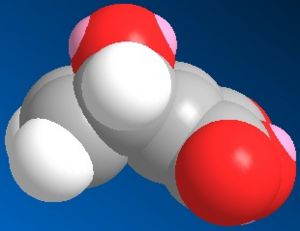
|
| Energy: 10.73 Kcal/mol | Energy: 67.35 Kcal/mol |
Looking at the norbornadiene type reactant which shows good correlation to literature structures it is most likely that the pyrrole will be least hindered attacking from the top face above the anhydride, it also has to be considered that there is likely to be a favourable hydrogen bonding reaction H----O if it approaches above.
The MO's can be calculated for the reactants and are shown below:
| Orbital | Pyrrole | Norborndiene Derivative |
|---|---|---|
| HOMO | 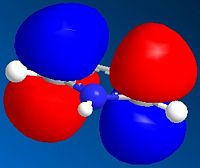 |
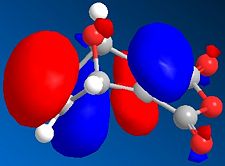
|
| LUMO | 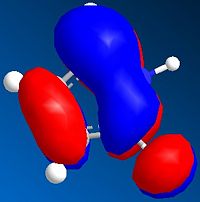 |
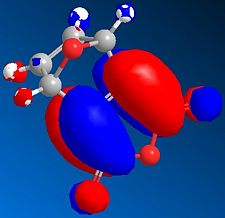
|
The HOMO orbitals reveals high electron density on the double bonds of both species and in particular high density on both double bonds of the norborndiene derivative suggesting both could be nucleophilic. The double bond connected to the anhydride however is selectively reacted in the Diels-Alder because the LUMO is positioned over that the double bond and the Diels-Alder will requires donation into the LUMO of the diene and the alkene.
The two products are based on the orientation of the pyrrole ring in the attack (i.e. N towards or away from the bridging oxygen). This is shown below:
| Exo,Exo-Diels Alder | Endo,Endo-Diels Alder |
|---|---|
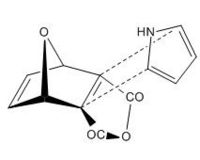 |
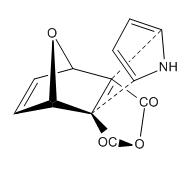
|
The Diels-Alder reaction of the above situations results in the two predicted products.
The two products can be told apart by their 13C NMR, Proton NMR, NOE NMR and X-Ray Crystal Structure. The first two can be predicted simply using computational techniques and will be addressed in this project.
IR spectrum's although easily computed were useless in distinguishing between isomers as the major vibration involves the C=O's and the spectrums observed were almost identical.
The 13C NMR was predicted using Gaussian and compared to the literature. The literature 13C NMR however is only given for the exo,exo syn nitrogen, oxygen bridging isomer as that was the experimental observed single adduct.
| Exo,exo | Exo,endo |
|---|---|
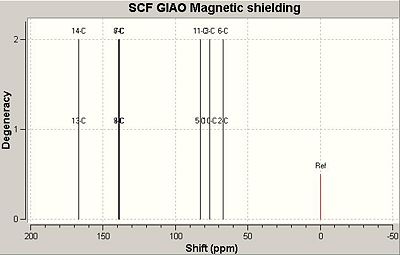 |
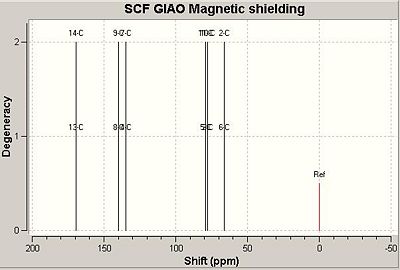
|
The key initial observation is the significant difference in the carbons shifts for the two C=C bonds in the exo,endo product where as fairly identical environments for the exo,exo product. This makes logical sense as with the two heterocycle bridges syn (up), the alkenes are both on the outside and at similar angles to the anhydride. The nitrogen and the oxygen bridges are likely to have similar effects. The exo,endo product however put the two alkene's in significantly different environments with the double bond now in between a nitrogen and an oxygen and antiperiplaner to the anhydride.
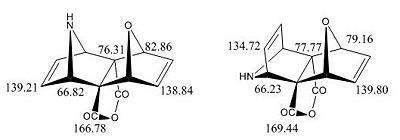
The NMR's are assigned as per diagram and table below, (N.B. Only 1 label given for identical carbons):
|
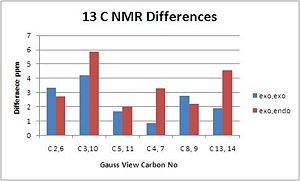
|
The exo,exo adduct shows quite closer correlation to the literature values recorded than the exo,endo product suggesting that the isomer suggested was the one obtained however due to the error in calculations and only a small difference it is not completely conclusive.
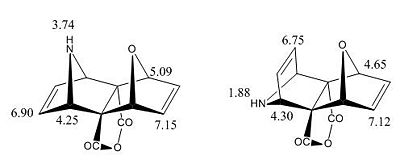
The H NMR was also modelled to help identify the isomer experimentally obtained the H NMR peaks obtained are recorded below:
|
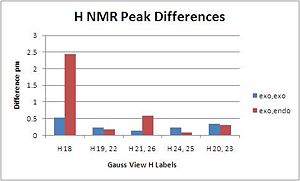
|
The H NMR recorded although reported to generally be quite inaccurate shows fairly close correlation to the observed spectrum, the big difference and relevant peak is the N-H proton (4.32) which is significantly different and matches well for the exo,endo product but over 1.5ppm out for the exo,endo product
All together the evidence suggests that the assignment of the exo,exo product for the reaction is correct however the 13C and H NMR methods are probably not the best methods to determine the isomers and crystal structures are probably more definite.
More complicated modelling involving modelling the transition state would be ideal to further identify the reasoning for the exo,exo adduct dominating over the exo,endo adduct
References
- ↑ [1] A. G. Shultz, L. Flood and J. P. Springer, J. Org. Chemistry, 1986, 51, 838.
- ↑ [2] S. Leleu, C.; Papamicael, F. Marsais, G. Dupas, V.; Levacher, Vincent. Tetrahedron: Asymmetry, 2004, 15, 3919-3928.
- ↑ [3] Alan B. McEwent and Paul von Rague Schleyer, J. Am. Chem. Soc., 1986, 108, 3951-3960.
- ↑ [4] B. Halton, R. Boese and H. S. Rzepa., J. Chem. Soc., Perkin Trans 2, 1992, 447
- ↑ [5] D. Margtic, R. N. Warrener, G. Sun and D. N. Butlery, Tetrahedron, 2007, 63, 4337-4346.
Note: selected pictures of reactions and compounds taken from mod:organic (i.e. Lab Page)