Dimethylmercury
Dimethylmercury
Dimethylmercury (CH3)2Hg belongs to the organometallic family of compounds (specifically the alkyl mercuries. It has a linear structure like many HgX2 systems. It is a liquid at room temperature and is flamable, colourless and described as having a sweet smelling odour.
Dimethylmercury is one of the most potent neurotoxins known. Absorption of less than 1ml can be serverely toxic[1]. The fact that many materials including plastics and rubbers, specifically latex and neoprene are permeable to the compound, makes it even more deadly. Also its high vapour pressure (50-82mmHg @ 293K)[2] makes inhalation a significant mode of entry. The recommended handling advice for DMM is to wear a face shield at least 8 inches long with impervious gloves (silver shield laminate gloves have been shown to remain impervious to DMM for at least 4 hours)
Uses
Dimethylmercury is primarily used in research. A major use is in the calibration of NMR machines. Although due to the highly toxic nature of the compound, inorganic Hg salts are prefered.
Synthesis
One means of producing DMM is by the oxidation of monomethyl hydrazine by mercuric oxide)[3] as shown below:
2CH3NHNH2 + HgO = C2H6Hg + NH3 + NO
Neurotoxicity
Dimethylmercury crosses the blood brain barrier rapidly due to the formation of a methyl-mercury cysteine complex. Hg is a soft acid and will therefore bind to soft bases (eg Sulphur). Thus Hg will attack the thiol groups of enzymes causing their inhibition. The removal of the compound from the body occurs over a long period thus it will tend to bioaccumulate in the body. Such that, often, by the time effects are seen the (fatal) damage has been done. Symptoms of DMM poisoning include ataxia, sensory disturbances and changes in mental state.
Death of a scientist

One of the most famous cases involving DMM poisoning is that of Karen Wetterhahn, who died as a result of exposure to the compound. Wetterhahn, an internationally respected professor of chemistry at Dartmouth College, New Hampshire, was using DMM as a Hg NMR standard reference for her study of the interaction of Hg ions with DNA repair proteins. In January 1997 she reported symptoms such as slurred speech and tingling fingers and toes, all consistent with Hg poisoning. She later recalled spilling a single drop of DMM onto her latex gloved hand. On 28th January of that year she was officially diagnosed with Hg poisoning. Her blood-Mercury level was found to be 4000 micrograms per litre, 80 times the toxic threshold. Despite aggressive chelation treatment she died on 8th June 1997 after falling into a coma. The official verdict was death by encephalopathy as a result of mercury intoxication.
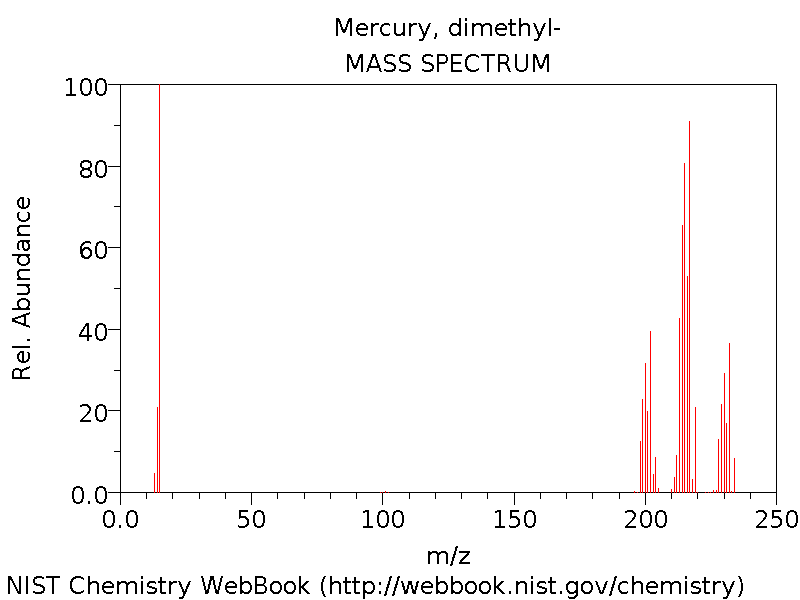
Spectra
The Mass Spectrum[4] is shown below:
References
- ↑ Blyney MB, JS Winn and DW Nierenberg, 'Letters; chemical safety - handling of dmHg'. Chem and Engineering news (1997, May, 12); 7.
- ↑ [1]Occupational Safety and Health Administration, US Department of Labor.
- ↑ Harry H Sisler, Milap A Mathur, Sampat R Jain and Roy W King. J Org Chem 1980, 45, 1329-1330.
- ↑ [2]NIST Chemistry WebBook.
External Links
| Dimethylmercury | |||
|---|---|---|---|
| |||
| General | |||
| Systematic name | Dimethylmercury | ||
| Molecular formula | C2H6Hg | ||
| Molar mass | 230.659 g/mol | ||
| CAS number | 593-74-8 | ||
| Properties | |||
| Density & phase | 2.961 g/cm³ | ||
| Melting point | 270 K | ||
| Boiling point | 391-392 K | ||